Introduction. chemical energy into electrical energy by means of redox reactions.
|
|
|
- Amos Copeland
- 5 years ago
- Views:
Transcription
1 CHAPTER I
2 Introduction 1. 1 Battery An electrical battery is one or more electrochemical cells that convert stored chemical energy into electrical energy by means of redox reactions History of batteries 1748 Benjamin Franklin first coined the term "battery" to describe an array of charged glass plates Luigi Galvani was dissecting a frog affixed to a brass hook. When he touched its leg with his iron scalpel, the leg twitched. Galvani believed the energy that drove this contraction came from the leg itself, and called it "animal electricity" Volta invented the first true battery which came to be known as the Voltaic Pile. It consisted of pairs of copper and zinc discs piled on top of each other, separated by a layer of cloth or cardboard soaked in brine (i.e. the electrolyte) John Frederic Daniell invented the Daniell cell, which consisted of a copper pot filled with a copper sulphate solution, in which was immersed an unglazed earthenware container filled with sulphuric acid and a zinc electrode William Robert Grove invented Grove cell. It consisted of a zinc anode dipped in sulfuric acid and a platinum cathode dipped in nitric acid, separated by porous earthenware. The Grove cell provided a high current and nearly twice the voltage of the Daniell cell. 1
3 1859 Gaston Plante invented the lead acid battery, the first ever battery that could be recharged by passing a reverse current through it. A lead acid cell consists of a lead anode and a lead dioxide cathode immersed in sulphuric acid Georges Leclanche invented a battery that consisted of a zinc anode and a manganese dioxide cathode wrapped in a porous material, dipped in a jar of ammonium chloride solution. The manganese dioxide cathode had a little carbon mixed into it as well, which improved electrolyte conductivity and absorption Carl Gassner invented the first commercially successful dry cell battery (zinc carbon cell) Waldemar Jungner invented the nickel cadmium battery Thomas Alva Edison invented the alkaline storage battery Jungner invented the nickel iron battery Lithium battery 1989 Nickel/Metal hydride battery An American chemist John B. Goodenough led a research team at Sony that would produce the lithium ion battery, a rechargeable and more stable version of the lithium battery; the first ones were sold in lithium ion polymer battery. 2
4 1. 3 Categories and types of batteries Batteries are classified into two broad categories. Primary batteries irreversibly (within limits of practicality) transform chemical energy to electrical energy. When the initial supply of reactants is exhausted, energy cannot be readily restored to the battery by electrical means. eg. Zinc carbon batteries, Alkaline batteries Secondary batteries can be recharged; that is, they can have their chemical reactions reversed by supplying electrical energy to the cell, restoring their original composition. eg. Lead acid batteries, Lithium ion batteries 1. 4 Battery cell types There are many general types of electrochemical cells, according to chemical processes applied and designs chosen. The variation includes galvanic cells, electrolytic cells, fuel cells, flow cells and voltaic piles Wet cell A wet cell battery has a liquid electrolyte. Other name is flooded cell, since the liquid covers all internal parts, or vented cell, since gases produced during operation can escape to the air. Wet cells may be primary cells (non rechargeable) or secondary cells (rechargeable). eg. Leclanche cell. 3
5 Dry cell A dry cell has the electrolyte immobilized as a paste, with only enough moisture in the paste to allow current to flow. Compared to a wet cell, the battery can be operated in any random position, and will not spill its electrolyte if inverted. eg. Zinc carbon battery Molten salt battery A molten salt battery is a primary or secondary battery that uses a molten salt as its electrolyte. eg. ZEBRA batteries (Na NiCl 2 battery) Reserve battery A reserve battery can be stored for a long period of time and is activated when its internal parts (usually electrolyte) are assembled. For example, a battery for an electronic fuze might be activated by the impact of firing a gun, breaking a capsule of electrolyte to activate the battery and power the fuze's circuits. eg. Water activated battery Components of cell Anode or negative electrode Cathode or positive electrode Electrolyte Separator Current collector Anode The electrode at which the oxidation occurs is called the anode. The charge on the anode is negative. 4
6 Characteristics Efficiency as a reducing agent. High coulombic output (Ah/g) Good conductivity. Stability Example Metals Cathode The electrode at which reduction occurs is termed the cathode. The charge on the cathode is positive. Characteristics Good oxidizing agent. Must stable when contact with electrolyte. To achieve high performance. Should be conductive. Example Metallic oxides Electrolyte The ionic conductor which provides the medium for transfer of charge, as ions, inside the cell between the anode and cathode. Characteristics Good ionic conductivity Low viscosity and high dielectric constant 5
7 Non reactivity with electrode materials Example Sulphuric acid, LiPF 6 in 1: 1 (Ethylene carbonate and diethylene carbonate) Separator An ion permeable and electronically non conductive, spacer or material which prevents electronic contact between electrodes of opposite polarity in the same cell. Characteristics Chemical stability. Low thickness. Mechanical strength. High porosity & permeability. Examples Polypropylene, Glass, Non Woven glass Current collector An inert member of high electrical conductivity used to conduct current from or to an electrode during discharge or charge. Characteristics Excellent bulk electrical conductivity. Minimal thickness / weight. Excellent surface conductivity. Examples Aluminium foil, Copper foil and Nickel mesh. 6
8 1. 6 Operating principle of cell Discharge The operation of a cell during discharge is shown schematically in Fig. 1. 1a. When the cell is connected to an external load, electrons flow from the anode, which is oxidized, through the external load to the cathode, where the electrons are accepted and the cathode material is reduced. The electric circuit is completed in the electrolyte by the flow of anions (negative ions) and cations (positive ions) to the anode and cathode, respectively Charge During the recharge of a rechargeable or storage cell, the current flow is reversed and oxidation takes place at the positive electrode and reduction at the negative electrode, as shown in Fig. 1. 1b. As the anode is, by definition, the electrode at which oxidation occurs and the cathode, the one where reduction takes place, the positive electrode is now the anode and the negative the cathode Lithium batteries Lithium batteries 1 were first proposed by M. S. Whittingham at Binghamton University, at Exxon, in the 1970s. Whittingham used titanium (II) sulfide as the cathode and lithium metal as the anode. The reversible intercalation in graphite and intercalation into cathodic oxides was also already discovered 2 in the 1970s by J.O. Besenhard at TU Munich. He also proposed the application as high energy density lithium cells. In 1979, John Goodenough demonstrated a rechargeable 3 cell with high cell voltage in the 4V range using lithium cobalt oxide (LiCoO 2 ) as the positive electrode and lithium metal as the negative electrode. This innovation provided the positive electrode material which 7
9 made lithium ion batteries (LIBs) possible. LiCoO 2 is a stable positive electrode material which acts as a donor of lithium ions, which means that it can be used with a negative electrode material other than lithium metal. In 1985, Akira Yoshino 4 assembled a prototype cell using carbonaceous material into which lithium ions could be inserted as the anode, and as the cathode lithium cobalt oxide (LiCoO 2 ), which is stable in air. By using an anode material without metallic lithium, safety was dramatically improved over batteries which used lithium metal. The use of lithium cobalt oxide (LiCoO 2 ) enabled industrial scale production to be achieved easily. This was the birth of the current lithium ion battery. In 1991, Sony and Asahi Kasei released the first commercial lithium ion battery Advantages of lithium ion battery High voltage Lithium cells have voltages up to about 4V, depending on the cathode material, compared with 1.5V for most other primary system. High open circuit voltage in comparison to aqueous batteries (such as lead acid, nickel metal hydride and nickel cadmium). This is beneficial because it increases the amount of power that can be transferred at a lower current. High energy density and power density The energy density ( Wh/Kg) and power density (250 Wh/L) of lithium cell is 2 4 or more times better than that of conventional batteries (Lead acid, zinc anode and Ni/MH batteries). 8
10 Operation over a wide temperature range Many of the lithium cells will perform over a temperature range from 40 to 70 C. Flat discharge characteristics A flat discharge curve is typical for many lithium cells. No memory effect Superior shelf life Self discharge rate of approximately 5 10% per month, compared to over 30% per month in common nickel metal hydride batteries, approximately 1.25% per month for low self discharge Ni MH batteries and 10% per month in nickel cadmium batteries Electrochemistry of lithium ion Battery During charge, the positive material is oxidized and the negative material is reduced. In this process, lithium ions are de intercalated from the positive material and intercalated into the negative material. (Intercalated a reaction where lithium ions are reversibly removed or inserted into a host without a significant structural change to the host) The reverse process is present during a discharge cycle. The operation of a Lithium ion cell is shown schematically in Fig The positive electrode half reaction is Charge LiCoO 2 Li 1 x CoO 2 + xli + + xe Discharge 9
11 The negative electrode half reaction is Charge xli + + xe + 6C Li x C 6 Discharge The over all electrode reaction is Discharge CoO 2 + LiC 6 LiCoO 2 + C 6 Charge Classification of lithium batteries Lithium batteries are classified into two broad categories. Lithium battery Li primary battery Li secondary battery Soluble cathode cells Solid cathode cells Solid electrolyte cells Lithium ion battery Lithium ion polymer battery Classification of lithium primary battery material. Lithium primary cells classified on the basis of electrolyte used or cathode 10
12 a. Soluble cathode cells These use liquid or gaseous cathode materials that dissolve in the electrolyte or the electrolyte solvent. Their operation depends on the formation of a passive layer on the lithium anode resulting from a reaction between the lithium and the cathode material. This prevents further chemical reaction (self discharge) between anode and cathode or reduces it to a very low rate. These cells are manufactured in many different configurations and designs (such as high and low rate) and with a very wide range of capacities. They are generally fabricated in cylindrical configuration in the smaller sizes, upto about 35 Ah, using a bobbin construction for the low rate cells and a spirally wound (jelly roll) structure for the high rate designs. Prismatic containers, having flat parallel plates, are generally used for the larger cells upto 10,000 Ah in size. Flator pancake shaped configurations have also been designed. These soluble cathode lithium cells are used for low to high discharge rates. The high rate designs, using large electrode surface areas, are noted for their high power density and are capable of delivering the highest current densities of any active primary cell. Eg. Li/SO 2 Cl 2 cells b. Solid cathode cells The second type of lithium anode primary cells uses solid rather than soluble gaseous or liquid materials for the cathode. With these solid cathode materials, the cells have the advantage of not being pressurized or necessarily requiring a hermetic type seal, but they do not have the high rate capability of the soluble cathode systems. They are designed, generally, for low to medium rate applications such as memory backup, security devices, portable electronic equipment, photographic equipment, watches, 11
13 calculators and small lights. Button, flat, and cylindrical shaped cells are available in low rate and the moderate rate jelly roll configurations. Eg. Li/V 2 O 5 cell. c. Solid electrolyte cells The third type of lithium anode primary batteries uses solid rather than liquid for electrolytes. The solid electrolyte is formed in situ as the discharge product of the cell reaction. However, the viscous liquid phase impart a plasticity to the cathode which makes these solid state cells better able to adapt to volumetric changes during cell discharge. These cells are noted for their extremely long storage life, in excess of 20 years, but are capable of only low rate discharge in the micro ampere range. They are used in applications such as memory backup, cardiac pacemakers, and similar equipment where current requirements are low but long life is critical. Eg. Li/LiI/I 2 (P 2 VP) Classification of lithium secondary batteries Lithium secondary cells classified on the basis of electrolyte used. a. Lithium ion battery The three primary functional components of a lithium ion battery are the anode, cathode, and electrolyte. The anode of a conventional lithium ion cell is made from carbon, the cathode is a metal oxide, and the electrolyte is a lithium salt in an organic solvent. The most commercial popular anode material is graphite. The cathode is generally one of three materials: a layered oxide (such as lithium cobalt oxide), a polyanion (such as lithium iron phosphate), or a spinel (such as lithium manganese oxide). The electrolyte is typically a mixture of organic carbonates such as ethylene carbonate or diethyl carbonate containing complexes of lithium ions. These non aqueous 12
14 electrolytes generally use non coordinating anion salts such as lithium hexafluorophosphate (LiPF 6 ), lithium hexafluoroarsenate monohydrate (LiAsF 6. H 2 O), lithium perchlorate (LiClO 4 ), lithium tetrafluoroborate (LiBF 4 ), and lithium triflate (LiCF 3 SO 3 ). Depending on materials choices, the voltage, capacity, life, and safety of a lithium ion battery can change dramatically. b. Lithium ion polymer battery Lithium ion polymer batteries, polymer lithium ion, or more commonly lithium polymer batteries (abbreviated Li poly, Li Pol, LiPo, LIP, PLI or LiP) are rechargeable batteries (secondary cell batteries). This battery consists of a carbon anode and Li + insertion material cathode and solid polymer electrolyte, in which lithium ions swing between the two electrodes. Lithium polymer batteries have the same basic chemistry as lithium ion batteries. However, the polymer cells use a porous separator that, when exposed to the electrolyte, turns to a gel because the gel isn't flammable, lithium polymer batteries have a different architecture, with the anode and cathode developed as a plate and stacked on top of each other. Polymer electrolytes/separators can be solid polymers (e.g., polyethylene oxide) and LiPF 6, or other conducting salts and SiO 2, or other fillers for better mechanical properties. Lithium polymer batteries do not need a metal shell the way that lithium ion batteries do. In fact, the shell of lithium polymer batteries is often plastic Components of lithium ion cells Anode The anode is the electrode at which oxidation takes place and electrons are fed into the external circuit. Different type of anode materials are used in currently used 13
15 lithium ion batteries such as lithium metal, carbonaceous materials, Sn, SnO based materials and Intermetallic alloys etc., Criteria The potential of lithium insertion and deinsertion in the anode Vs Li must be as low as possible. The amount of lithium which can be accommodated by the anode material should be as high as possible to achieve a high specific capacity. The anode should endure repeated Li insertion and deinsertion without any structural change to obtain long cycle life. Examples. Li, graphite, metal and metal oxides Cathode The cathode is the electrode at which reduction takes place and into which electrons are fed from the external circuit. A guest spaced such as lithium can be inserted interstitially into the host lattice and extracted during recharge with little or no structural modification of the host. The intercalated compounds are classified as follows LiMO 2 based materials (M = Co, Ni, Mn) LiMPO 4 based materials (M = Fe, Co, Ni and Mn) LiMn 2 O 4 based materials Criteria The insertion compound Li x M y X z (X anion) should have a high lithium chemical potential (µ Li(c) ) to maximize the cell voltage. The insertion compound Li x M y X z should allow an insertion/extraction of a large amount of lithium, to maximize the cell capacity. 14
16 The lithium insertion/extraction process should be reversible with no or minimal changes in the host structure over the entire range x of lithium insertion/extraction in order to provide a good cycle life for the cell. The insertion compound should support mixed conduction. It should have good electronic conductivity σ e and lithium ion conductivity σ Li to minimize polarization losses during the discharge/charge process and thereby to support a high current density and power density. The insertion compound should be chemically stable without undergoing any reaction with the electrolyte over the entire range, x of lithium insertion/extraction. Examples. LiCoO 2, LiMn 2 O 4, LiFePO 4 etc Electrolyte Electrolytes can be defined as to serve as the medium for the transfer of charges, which are in the form of ions, between a pair of electrodes. The vast majority of the electrolytes are electrolytic solution types that consists of salts dissolved in either water (aqueous) or organic molecules (nonaqueous) and are in a liquid in the service temperature range. Criteria It should be a good ionic conductor and electronic insulator It should have wide electrochemical window It should also be inert to other cell components It should be robust against other various abuses, such as electrical, mechanical or thermal ones. 15
17 Its components should be environmentally friendly. Classification Electrolytes can be roughly divided into three groups as follows Liquid electrolytes eg. LiPF 6 in 1: 1 (EC: DEC) Solid polymer electrolyte eg. LiClO 4 PEO Gel polymer electrolyte eg. PVDF HFP Separators A separator is a porous membrane placed between electrodes of opposite polarity, permeable to ionic flow but preventing electrical contact of the electrodes. Criteria Electronic insulator Minimal electrolyte (ionic) resistance Mechanical and dimensional stability Sufficient physical strength to allow easy handling Chemical resistance to degradation by electrolyte, impurities and electrode reactants and products Effective in preventing migration of particles or colloidal or soluble species between the two electrodes Readily wetted by electrolyte Uniform in thickness and other properties 16
18 Classification Separators for batteries can be divided into different types, depending on their physical and chemical characteristics. They can be molded, woven, non oven, microporous, bonded, papers, or laminates. Eg. Polypropylene, cellulose, nonwoven fabric and celgard etc Current collectors A structural part of a complicated electrode assembly. Its primary purpose is to conduct the electricity between the actual working (reacting) parts of the electrode and the terminals. Current collectors must be electrochemically stable when in contact with the cell component during the potential operation window of an electrode. In lithium batteries Al can be used as current collector for positive electrode and Cu foil for negative electrode. The rough surface of substrate enhanced the adhesive force between an active material and a current collector. Therefore, surface roughness of substrate is an important factor to improve the cycleability of Li ion cell. a. Aluminium foil For high voltage 5 (>3.5 V Vs Li/Li + ) LIBs, Al is the material of choice. It is used extensively with lithiated transition metal oxides upto 5V Vs. Li/Li +. In air and aqueous solutions, Al can be protected by a thin and dense oxide passive layer, Al 2 O 3. Its low price and good electrical conductivity due to a high purity of Al metal expand the potential application for lithium batteries. b. Copper foil Almost all commercial, rechargeable lithium batteries use carbonaceous materials applied to a copper foil substrate as the negative electrode. As lithium ions, which are 17
19 released from the positive electrodes, are intercalated to the carbonaceous negative electrode materials, the resulting potential 6 reaches between 0.25 and 0.01 V Vs. Li/Li +. In this state, the negative electrode materials, the Cu current collector, and the electrolyte are electrochemically reduced. Cu metal surface is likely to reduce the electrolyte at the potential 3 V Vs. Li/Li +, generating the cathodic current beecause of this, Cu metal is stable at a lower narrow potential range and is generally acceptable for negative electrode current collectors Applications of lithium primary and secondary batteries Lithium primary and secondary batteries are widely used in consumer, industrial, medical, automotive and military devices. General usage Lithium ion batteries can be used both in devices that need recharging, such as cell phones, and in products whose batteries are difficult, expensive or impossible to recharge or replace, such as cardiac pacemakers. Portable electronics In portable electronics, batteries needs to be recharged many times, and the lithium ion battery can handle hundreds of recharges. Products that use the lithium ion battery include ipods, cell phones, PCs, laptops, watches and digital cameras. Medical applications Implantable electronic devices cannot be recharged or replaced without great expense, so the batteries used need to be small and able to last for years. Implantable products that use lithium ion batteries include cardiac pacemakers, cardiac defibrillators, neuro stimulators and drug infusion systems. 18
20 Military applications The lithium ion battery's long life and light weight make it the battery used in many military functions, including providing power for the computers in missiles. 19
21 1. 13 References 1. M. S. Whittingham, Science 192 (1976) R. Schallhorn, R. Kuhlmann, J. O. Besenhard, Mater. Res. Bull. 11 (1976) USPTO search for inventions by "Goodenough, John". 4. US , Yoshino; Akira, "Secondary Battery", issued 10 May 1985, assigned to Asahi Kasei. 5. S. T. Myung, Y. Hitoshi, Y. K. Sun, J. Mater. Chem. 21 (2011) K. L. Lee, J. Y. Jung, S. W. Lee, H. S. Moon, J. W. Park, J. Power Sources 129 (2004)
22 Fig Operation of cell a) Discharge b) Charge Fig Lithium ion battery operating principle (source: 21
A Structure of Cylindrical Lithium-ion Batteries
 Introduction A Structure of Cylindrical Lithium-ion Batteries A lithium-ion battery is an energy storage device providing electrical energy by using chemical reactions. A few types of lithium-ion battery
Introduction A Structure of Cylindrical Lithium-ion Batteries A lithium-ion battery is an energy storage device providing electrical energy by using chemical reactions. A few types of lithium-ion battery
Li-ion Technology Overview NTSB Hearing Washington, D.C. July 12-13, 2006
 Li-ion Technology Overview NTSB Hearing Washington, D.C. July 12-13, 2006 Jason Howard, Ph.D. Distinguished Member of the Technical Staff, Motorola, Inc. Board of Directors, Portable Rechargeable Battery
Li-ion Technology Overview NTSB Hearing Washington, D.C. July 12-13, 2006 Jason Howard, Ph.D. Distinguished Member of the Technical Staff, Motorola, Inc. Board of Directors, Portable Rechargeable Battery
Lithium Ion Batteries - for vehicles and other applications
 Lithium Ion Batteries - for vehicles and other applications Tekes 2008-12-03 Kai Vuorilehto / European Batteries What do we need? High energy (Wh/kg) driving a car for 5 hours High power (W/kg) accelerating
Lithium Ion Batteries - for vehicles and other applications Tekes 2008-12-03 Kai Vuorilehto / European Batteries What do we need? High energy (Wh/kg) driving a car for 5 hours High power (W/kg) accelerating
Duracell Battery Glossary
 Duracell Battery Glossary 1 Duracell Battery Glossary AB Absorption Alloy Ambient Humidity Ambient Temperature Ampere-Hour Capacity Anode Battery or Pack Bobbin C-Rate (also see Hourly Rate) Capacity Capacity
Duracell Battery Glossary 1 Duracell Battery Glossary AB Absorption Alloy Ambient Humidity Ambient Temperature Ampere-Hour Capacity Anode Battery or Pack Bobbin C-Rate (also see Hourly Rate) Capacity Capacity
GLOSSARY: TECHNICAL BATTERY TERMS
 GLOSSARY: TECHNICAL BATTERY TERMS AB5 Absorption Alloy Ambient Humidity Ambient Temperature Ampere-Hour Capacity Anode Battery or Pack Bobbin C-Rate (also see Hourly Rate) Capacity Capacity Retention (or
GLOSSARY: TECHNICAL BATTERY TERMS AB5 Absorption Alloy Ambient Humidity Ambient Temperature Ampere-Hour Capacity Anode Battery or Pack Bobbin C-Rate (also see Hourly Rate) Capacity Capacity Retention (or
CELLS AND BATTERIES Understand the general features of cells and batteries Describe the relationship between cells and batteries. Describe the basic
 Cell & Batteries CELLS AND BATTERIES Understand the general features of cells and batteries Describe the relationship between cells and batteries. Describe the basic operation of a battery. Compare between
Cell & Batteries CELLS AND BATTERIES Understand the general features of cells and batteries Describe the relationship between cells and batteries. Describe the basic operation of a battery. Compare between
UN/SCETDG/47/INF.13/Rev.1
 Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification and Labelling of Chemicals New proper shipping name for rechargeable lithium metal batteries
Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification and Labelling of Chemicals New proper shipping name for rechargeable lithium metal batteries
Battery Power for All-Electric Road Vehicles John B. Goodenough and M. Helena Braga The University of Texas at Austin, and of Porto, Portugal
 Battery Power for All-Electric Road Vehicles John B. Goodenough and M. Helena Braga The University of Texas at Austin, and of Porto, Portugal Modern Society runs on the energy stored in fossil fuels. This
Battery Power for All-Electric Road Vehicles John B. Goodenough and M. Helena Braga The University of Texas at Austin, and of Porto, Portugal Modern Society runs on the energy stored in fossil fuels. This
Lithium battery knowledge
 Seminar on Safe Transport of Lithium Battery by Air Lithium battery knowledge 12 December 2008 At Cathay City s s Auditorium Battery Association of Japan(BAJ) 1 Seminar on Safe Transport of Lithium Battery
Seminar on Safe Transport of Lithium Battery by Air Lithium battery knowledge 12 December 2008 At Cathay City s s Auditorium Battery Association of Japan(BAJ) 1 Seminar on Safe Transport of Lithium Battery
Congratulations, Dorothy!
 Congratulations, Dorothy! Battery Overview Steve Garland Kyle Jamieson Outline Why is this important? Brief history of batteries Basic chemistry Battery types and characteristics Case study: ThinkPad battery
Congratulations, Dorothy! Battery Overview Steve Garland Kyle Jamieson Outline Why is this important? Brief history of batteries Basic chemistry Battery types and characteristics Case study: ThinkPad battery
Course of development of the lithium-ion battery (LIB), and recent technological trends
 Session 2A : Business Case Course of development of the lithium-ion (LIB), and recent technological trends Dr. Akira Yoshino Yoshino Laboratory Asahi Kasei Corp. E-mail: yoshino.ab@om.asahi-kasei.co.jp
Session 2A : Business Case Course of development of the lithium-ion (LIB), and recent technological trends Dr. Akira Yoshino Yoshino Laboratory Asahi Kasei Corp. E-mail: yoshino.ab@om.asahi-kasei.co.jp
Introduction. Today, we can convert energy from many different forms into usable electricity.
 Introduction Today, we can convert energy from many different forms into usable electricity. But how did we get here? In ancient times, the generation of electricity was purely accidental. 1. Drag feet
Introduction Today, we can convert energy from many different forms into usable electricity. But how did we get here? In ancient times, the generation of electricity was purely accidental. 1. Drag feet
UN/SCETDG/52/INF.11. Sodium-Ion Batteries. Introduction
 Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification and Labelling of Chemicals UN/SCETDG/52/INF.11 Sub-Committee of Experts on the Transport
Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification and Labelling of Chemicals UN/SCETDG/52/INF.11 Sub-Committee of Experts on the Transport
New proper shipping name for rechargeable lithium metal batteries
 Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification and Labelling of Chemicals New proper shipping name for rechargeable lithium metal batteries
Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification and Labelling of Chemicals New proper shipping name for rechargeable lithium metal batteries
Energy Storage. 3. Batteries. Assoc. prof. Hrvoje Pandžić. Ivan Pavić, MEE Vedran Bobanac, PhD
 Energy Storage 3. Batteries Assoc. prof. Hrvoje Pandžić Ivan Pavić, MEE Vedran Bobanac, PhD 1 Batteries - definition Electrochemical devices Potential difference between two different metals submerged
Energy Storage 3. Batteries Assoc. prof. Hrvoje Pandžić Ivan Pavić, MEE Vedran Bobanac, PhD 1 Batteries - definition Electrochemical devices Potential difference between two different metals submerged
There are several technological options to fulfill the storage requirements. We cannot use capacitors because of their very poor energy density.
 ET3034TUx - 7.5.1 - Batteries 1 - Introduction Welcome back. In this block I shall discuss a vital component of not only PV systems but also renewable energy systems in general. As we discussed in the
ET3034TUx - 7.5.1 - Batteries 1 - Introduction Welcome back. In this block I shall discuss a vital component of not only PV systems but also renewable energy systems in general. As we discussed in the
THE BUSINESS CASE FOR INDUSTRIAL-SCALE BATTERIES
 11 THE BUSINESS CASE FOR INDUSTRIAL-SCALE BATTERIES TECHNOLOGY OVERVIEW Batteries store electricity as chemical energy so that it can be recovered for later use. There are many different battery types;
11 THE BUSINESS CASE FOR INDUSTRIAL-SCALE BATTERIES TECHNOLOGY OVERVIEW Batteries store electricity as chemical energy so that it can be recovered for later use. There are many different battery types;
Battery Power for the Future
 March/April 2008 www.batterypoweronline.com Volume 12, Issue 2 Battery Power for the Future Is the Energy Output of Batteries Reaching its Limit? David Linden and Thomas B. Reddy, Ph.D. Co-Editors Handbook
March/April 2008 www.batterypoweronline.com Volume 12, Issue 2 Battery Power for the Future Is the Energy Output of Batteries Reaching its Limit? David Linden and Thomas B. Reddy, Ph.D. Co-Editors Handbook
Batteries for electric commercial vehicles and mobile machinery
 Batteries for electric commercial vehicles and mobile machinery Tekes EVE annual seminar, Dipoli 6.11.2012 Dr. Mikko Pihlatie VTT Technical Research Centre of Finland 2 Outline 1. Battery technology for
Batteries for electric commercial vehicles and mobile machinery Tekes EVE annual seminar, Dipoli 6.11.2012 Dr. Mikko Pihlatie VTT Technical Research Centre of Finland 2 Outline 1. Battery technology for
Performance Characteristics
 Performance Characteristics 5.1 Voltage The nominal voltage of Li/M no 2 cells is 3. volts, twice that of conventional cells due to the high electrode potential of elemental lithium. Consequently a single
Performance Characteristics 5.1 Voltage The nominal voltage of Li/M no 2 cells is 3. volts, twice that of conventional cells due to the high electrode potential of elemental lithium. Consequently a single
Unit 13 Batteries and Other Electrical Sources
 Batteries and Other Electrical Sources Objectives: Discuss the differences between primary and secondary cells. List voltages for different types of cells. Discuss different types of primary cells. Construct
Batteries and Other Electrical Sources Objectives: Discuss the differences between primary and secondary cells. List voltages for different types of cells. Discuss different types of primary cells. Construct
The Inside Story of the Lithium Ion Battery. John Dunning, Research Scholar in Residence Daniel Forbes, Graduate Student Electrical Engineering
 The Inside Story of the Lithium Ion Battery John Dunning, Research Scholar in Residence Daniel Forbes, Graduate Student Electrical Engineering Outline Background - Why this is important Electrochemistry/Battery
The Inside Story of the Lithium Ion Battery John Dunning, Research Scholar in Residence Daniel Forbes, Graduate Student Electrical Engineering Outline Background - Why this is important Electrochemistry/Battery
Lithium-ion polymer battery
 1 of 5 4/18/2009 7:19 PM Lithium-ion polymer battery From Wikipedia, the free encyclopedia Lithium-ion polymer batteries, polymer lithium ion, or more commonly lithium polymer batteries (abbreviated Li-poly,
1 of 5 4/18/2009 7:19 PM Lithium-ion polymer battery From Wikipedia, the free encyclopedia Lithium-ion polymer batteries, polymer lithium ion, or more commonly lithium polymer batteries (abbreviated Li-poly,
Battery technologies and their applications in sustainable developments. Dr. Denis Y.W. Yu Assistant Professor School of Energy and Environment
 Battery technologies and their applications in sustainable developments Dr. Denis Y.W. Yu Assistant Professor School of Energy and Environment May 29, 2014 Energy flow Energy Energy generation Energy storage
Battery technologies and their applications in sustainable developments Dr. Denis Y.W. Yu Assistant Professor School of Energy and Environment May 29, 2014 Energy flow Energy Energy generation Energy storage
The BEEST: An Overview of ARPA-E s Program in Ultra-High Energy Batteries for Electrified Vehicles
 The BEEST: An Overview of ARPA-E s Program in Ultra-High Energy Batteries for Electrified Vehicles David Danielson, PhD Program Director, ARPA-E NDIA Workshop to Catalyze Adoption of Next-Generation Energy
The BEEST: An Overview of ARPA-E s Program in Ultra-High Energy Batteries for Electrified Vehicles David Danielson, PhD Program Director, ARPA-E NDIA Workshop to Catalyze Adoption of Next-Generation Energy
Cylindrical Primary Lithium Handbook and Application Manual
 : Energizer lithium iron disulfide differs from alkaline batteries in chemistry and construction. They are built in a spiral construction featuring two long, thin electrodes rolled together to form a jellyroll
: Energizer lithium iron disulfide differs from alkaline batteries in chemistry and construction. They are built in a spiral construction featuring two long, thin electrodes rolled together to form a jellyroll
ATTACHMENT FIVE. Universal Waste Packaging Guidelines
 ATTACHMENT FIVE Universal Waste Packaging Guidelines Guidelines for Packaging Straight Fluorescent Lamps 1. Lamps should be packaged in containers that protect the lamps during the storage and transport.
ATTACHMENT FIVE Universal Waste Packaging Guidelines Guidelines for Packaging Straight Fluorescent Lamps 1. Lamps should be packaged in containers that protect the lamps during the storage and transport.
HAWLEY George C. Hawley & Associates
 COMPARISON OF GRAPHITE ANODES WITH COMPETITORS GRAPHITE SUPPLY CHAIN 13-15 NOVEMBER 2016 ISLAND HOTEL NEWPORT BEACH CALIFORNIA USA GEORGE C. George Hawley was Research and Development Chemist at Morgan
COMPARISON OF GRAPHITE ANODES WITH COMPETITORS GRAPHITE SUPPLY CHAIN 13-15 NOVEMBER 2016 ISLAND HOTEL NEWPORT BEACH CALIFORNIA USA GEORGE C. George Hawley was Research and Development Chemist at Morgan
Batteries generally classifies into two main groups: primary and secondary battery types. Primary batteries are
 Battery types Batteries generally classifies into two main groups: primary and secondary battery types. Primary batteries are disposable batteries that cannot be recycled, and the secondary is the rechargeable
Battery types Batteries generally classifies into two main groups: primary and secondary battery types. Primary batteries are disposable batteries that cannot be recycled, and the secondary is the rechargeable
ELiTE Battery Information
 ELiTE Battery Information History of Li- Ion Batteries What is a Lithium-ion Battery? Two or more electrochemical cells, electrically interconnected. Each cell contains two electrodes and an electrolyte.
ELiTE Battery Information History of Li- Ion Batteries What is a Lithium-ion Battery? Two or more electrochemical cells, electrically interconnected. Each cell contains two electrodes and an electrolyte.
Industrial Batteries 101
 Industrial Batteries 101 SAFT, now proud part of the TOTAL Group* SAFT DEVELOPS AND MANUFACTURES ADVANCED-TECHNOLOGY BATTERY SOLUTIONS FOR MULTIPLE APPLICATIONS ON A GLOBAL SCALE Diversified base of industries
Industrial Batteries 101 SAFT, now proud part of the TOTAL Group* SAFT DEVELOPS AND MANUFACTURES ADVANCED-TECHNOLOGY BATTERY SOLUTIONS FOR MULTIPLE APPLICATIONS ON A GLOBAL SCALE Diversified base of industries
Batteries: Electricity though chemical reactions
 Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
GURUKUL. DG 002/15th September Dear Readers,
 GURUKUL DG 002/15th September 2008 Dear Readers, At the outset let us pray for the blessings of the Godess of Education. We are today publishing the History of Battery to start with. Let us proceed systematically
GURUKUL DG 002/15th September 2008 Dear Readers, At the outset let us pray for the blessings of the Godess of Education. We are today publishing the History of Battery to start with. Let us proceed systematically
Energy Storage. Electrochemical Cells & Batteries
 Energy Storage These notes cover the different methods that can be employed to store energy in various forms. These notes cover the storage of Electrical Energy, Kinetic Energy, and Pneumatic Energy. There
Energy Storage These notes cover the different methods that can be employed to store energy in various forms. These notes cover the storage of Electrical Energy, Kinetic Energy, and Pneumatic Energy. There
Primary lithium metal batteries from leading manufacturer EVE Battery
 dipl. ing. Zoltán Kiss Sales Endrich Bauelemente Vertriebs GmbH Primary lithium metal batteries from leading manufacturer EVE Battery T o find energizing solution for potable electronics devices is a challenge,
dipl. ing. Zoltán Kiss Sales Endrich Bauelemente Vertriebs GmbH Primary lithium metal batteries from leading manufacturer EVE Battery T o find energizing solution for potable electronics devices is a challenge,
Energy Storage (Battery) Systems
 Energy Storage (Battery) Systems Overview of performance metrics Introduction to Li Ion battery cell technology Electrochemistry Fabrication Battery cell electrical circuit model Battery systems: construction
Energy Storage (Battery) Systems Overview of performance metrics Introduction to Li Ion battery cell technology Electrochemistry Fabrication Battery cell electrical circuit model Battery systems: construction
DOE OVT Energy Storage R&D Overview
 DOE OVT Energy Storage R&D Overview David Howell Hybrid and electric vehicles, energy storage technologies and control systems National and international R&D-projects, research institutions and funding
DOE OVT Energy Storage R&D Overview David Howell Hybrid and electric vehicles, energy storage technologies and control systems National and international R&D-projects, research institutions and funding
FUEL CELLS AND BATTERIES LECTURE NO. 9
 SECONDARY BATTERIES Secondary or rechargeable batteries are widely used in many applications. The most familiar are starting, lighting, and ignition (SLI) automotive applications; industrial truck materials
SECONDARY BATTERIES Secondary or rechargeable batteries are widely used in many applications. The most familiar are starting, lighting, and ignition (SLI) automotive applications; industrial truck materials
FACETS OF GRAPHITE. June 2017
 FACETS OF GRAPHITE June 2017 1. INTRODUCTION What is Graphite? Why is Graphite Important? Current Demand & Prices for Selected High Purity Graphite Applications Contents 2. SELECTED APPLICATIONS Lithium
FACETS OF GRAPHITE June 2017 1. INTRODUCTION What is Graphite? Why is Graphite Important? Current Demand & Prices for Selected High Purity Graphite Applications Contents 2. SELECTED APPLICATIONS Lithium
The Insurance Institute of London
 The Insurance Institute of London CII CPD accredited - demonstrates the quality of an event and that it meets CII/PFS member CPD scheme requirements. This lecture and podcast count as 45 minutes of CPD
The Insurance Institute of London CII CPD accredited - demonstrates the quality of an event and that it meets CII/PFS member CPD scheme requirements. This lecture and podcast count as 45 minutes of CPD
Batteries for HTM. D. J. McMahon rev cewood
 Batteries for HTM D. J. McMahon 141004 rev cewood 2017-10-09 Key Points Batteries: - chemistry; know the characteristic cell voltages of common chemistries: NiCd/ NiMH 1.2V Hg 1.35V Zn Alkaline 1.5V Ag
Batteries for HTM D. J. McMahon 141004 rev cewood 2017-10-09 Key Points Batteries: - chemistry; know the characteristic cell voltages of common chemistries: NiCd/ NiMH 1.2V Hg 1.35V Zn Alkaline 1.5V Ag
Understanding Lithium-Ion Technology Jim McDowall (updated from Battcon 2008)
 Understanding Lithium-Ion Technology Jim McDowall (updated from Battcon 2008) PE/SB Winter Meeting 2015, New Orleans Background History Started with primary batteries with metallic lithium negatives True
Understanding Lithium-Ion Technology Jim McDowall (updated from Battcon 2008) PE/SB Winter Meeting 2015, New Orleans Background History Started with primary batteries with metallic lithium negatives True
Batteries: Stored Energy Discussion Questions:
 Batteries: Stored Energy Discussion Questions: 1) How is energy stored in a battery? 2) How many different types of batteries are there? 3) What kinds of tools and machinery can run on batteries? 4) Can
Batteries: Stored Energy Discussion Questions: 1) How is energy stored in a battery? 2) How many different types of batteries are there? 3) What kinds of tools and machinery can run on batteries? 4) Can
Use of Aqueous Double Layer Ultracapacitor using Hybrid CDI-ED Technology for the use in Hybrid Battery Systems
 Use of Aqueous Double Layer Ultracapacitor using Hybrid CDI-ED Technology for the use in Hybrid Battery Systems Overview By Robert Atlas, Aqua EWP,LLC. September 2007 Aqua EWP. has for the last 10 years
Use of Aqueous Double Layer Ultracapacitor using Hybrid CDI-ED Technology for the use in Hybrid Battery Systems Overview By Robert Atlas, Aqua EWP,LLC. September 2007 Aqua EWP. has for the last 10 years
consumer and industrial batteries. The differences between Battery design is rapidly evolving for both consumer and industrial applications.
 E n e r g y The differences between consumer and industrial batteries Battery design is rapidly evolving for both consumer and industrial applications. Edited by: Leslie Langnau, Managing Editor Consumer
E n e r g y The differences between consumer and industrial batteries Battery design is rapidly evolving for both consumer and industrial applications. Edited by: Leslie Langnau, Managing Editor Consumer
The Challenges of Electric Energy Storage. Nigel Taylor, Nick Green, Chris Lyness, Steve Nicholls
 The Challenges of Electric Energy Storage Nigel Taylor, Nick Green, Chris Lyness, Steve Nicholls Technology Walk Customer familiarity with recharging IC HEV PHEV EV Kinetic energy recovery Plug-in Battery
The Challenges of Electric Energy Storage Nigel Taylor, Nick Green, Chris Lyness, Steve Nicholls Technology Walk Customer familiarity with recharging IC HEV PHEV EV Kinetic energy recovery Plug-in Battery
BUTTON CELL CR2450S BRIEF SPECIFICATION
 BUTTON CELL CR2450S BRIEF SPECIFICATION Model: CR2450S Nominal Voltage: 3V Nominal Capacity:550mAh Standard Discharge with load: 15KΩ Weight: 6.8g Stainless steel container ISO9001 Certified UL Certified
BUTTON CELL CR2450S BRIEF SPECIFICATION Model: CR2450S Nominal Voltage: 3V Nominal Capacity:550mAh Standard Discharge with load: 15KΩ Weight: 6.8g Stainless steel container ISO9001 Certified UL Certified
Batteries for HTM. Basic Battery Parameters:
 Batteries for HTM Key Points Batteries: - chemistry; know the characteristic cell voltages of common chemistries: NiCd/ NiMH 1.2V Hg 1.35V Zn Alkaline 1.5V Ag Oxide 1.55V Pb 2.0V Li 3.0V LiIon/ LiPo 3.6V
Batteries for HTM Key Points Batteries: - chemistry; know the characteristic cell voltages of common chemistries: NiCd/ NiMH 1.2V Hg 1.35V Zn Alkaline 1.5V Ag Oxide 1.55V Pb 2.0V Li 3.0V LiIon/ LiPo 3.6V
Lithium-ion Batteries Material Strategy and Positioning. Energy Storage HARDWARE
 HARDWARE Energy Storage Lithium-ion Batteries Material Strategy and Positioning Lithium-ion batteries are to replace the nickel-metal hydride batteries that are currently being used in hybrid motor vehicles
HARDWARE Energy Storage Lithium-ion Batteries Material Strategy and Positioning Lithium-ion batteries are to replace the nickel-metal hydride batteries that are currently being used in hybrid motor vehicles
EE Chapter 2 Aircraft Storage Batteries
 EE 2145230 Chapter 2 Aircraft Storage Batteries Two types of batteries used on nearly all aircraft are nickel cadmium and lead acid batteries. All batteries produce dc voltage. 2.1 Dry Cells and Batteries
EE 2145230 Chapter 2 Aircraft Storage Batteries Two types of batteries used on nearly all aircraft are nickel cadmium and lead acid batteries. All batteries produce dc voltage. 2.1 Dry Cells and Batteries
Unit 13 Batteries and Other Electrical Sources
 Battery History Luigi Galvani in 1791 first noticed indications of electricity while experimenting with frog legs. Alessandro Volta in 1800 created the first practical battery. Batteries are composed of
Battery History Luigi Galvani in 1791 first noticed indications of electricity while experimenting with frog legs. Alessandro Volta in 1800 created the first practical battery. Batteries are composed of
Guidelines for Battery Electric Vehicles in the Underground
 Guidelines for Battery Electric Vehicles in the Underground Energy Storage Systems Rich Zajkowski Energy Storage Safety & Compliance Eng. GE Transportation Agenda Terminology Let s Design a Battery System
Guidelines for Battery Electric Vehicles in the Underground Energy Storage Systems Rich Zajkowski Energy Storage Safety & Compliance Eng. GE Transportation Agenda Terminology Let s Design a Battery System
Portable Power & Storage
 Portable Power & Storage NMTC Disruptive Technology Summit and TECH CONN3CT Workshops 28 April 2017 Edward J. Plichta Chief Scientist for Power & Energy Command Power & Integration Directorate Aberdeen
Portable Power & Storage NMTC Disruptive Technology Summit and TECH CONN3CT Workshops 28 April 2017 Edward J. Plichta Chief Scientist for Power & Energy Command Power & Integration Directorate Aberdeen
Talga Anode Enables Ultra-Fast Charge Battery
 ASX & Media Release 16 October 2018 ASX:TLG Talga Anode Enables Ultra-Fast Charge Battery New test results show Talga s lithium-ion battery anode product outperforming commercial benchmark and enabling
ASX & Media Release 16 October 2018 ASX:TLG Talga Anode Enables Ultra-Fast Charge Battery New test results show Talga s lithium-ion battery anode product outperforming commercial benchmark and enabling
Battery Seminar. Battery Technology Mid Term Forecast. Samuel De-Leon
 Shmuel De-Leon Energy Ltd. Where Knowledge and Vision Take Place Battery Seminar Battery Technology Mid Term Forecast Samuel De-Leon shmueld33@gmail.com 1 Proprietary Notice This document contains information
Shmuel De-Leon Energy Ltd. Where Knowledge and Vision Take Place Battery Seminar Battery Technology Mid Term Forecast Samuel De-Leon shmueld33@gmail.com 1 Proprietary Notice This document contains information
Development of battery materials with world s highest performance
 Tokyo University of Agriculture and Technology Nippon Chemi-Con Corporation May 6, 2010 Applying nano-hybrid technology to the next generation lithium-ion battery Development of battery materials with
Tokyo University of Agriculture and Technology Nippon Chemi-Con Corporation May 6, 2010 Applying nano-hybrid technology to the next generation lithium-ion battery Development of battery materials with
Li/CFx Batteries The Renaissance
 Li/CFx Batteries The Renaissance 1/3/2012 Shmuel De-Leon Shmuel De-Leon Energy, Ltd. www.sdle.co.il shmueld33@gmail.com Li-CFx The First Commercial Lithium Cells in the Market Main application: Lures for
Li/CFx Batteries The Renaissance 1/3/2012 Shmuel De-Leon Shmuel De-Leon Energy, Ltd. www.sdle.co.il shmueld33@gmail.com Li-CFx The First Commercial Lithium Cells in the Market Main application: Lures for
Use of Aqueous Double Layer Ultracapacitor using Hybrid CDI-ED Technology for the use in Hybrid Battery Systmes
 Overview Use of Aqueous Double Layer Ultracapacitor using Hybrid CDI-ED Technology for the use in Hybrid Battery Systmes By Robert Atlas, Aqua EWP,LLC. September 2006 Aqua EWP. has for the last 10 years
Overview Use of Aqueous Double Layer Ultracapacitor using Hybrid CDI-ED Technology for the use in Hybrid Battery Systmes By Robert Atlas, Aqua EWP,LLC. September 2006 Aqua EWP. has for the last 10 years
Enhancing the Reliability & Safety of Lithium Ion Batteries
 Enhancing the Reliability & Safety of Lithium Ion Batteries Over the past 20 years, significant advances have been made in rechargeable lithium-ion (Li-Ion) battery technologies. Li-Ion batteries now offer
Enhancing the Reliability & Safety of Lithium Ion Batteries Over the past 20 years, significant advances have been made in rechargeable lithium-ion (Li-Ion) battery technologies. Li-Ion batteries now offer
Introduction to Solar Electric Battery Systems. J-Tech Solar Training
 Introduction to Solar Electric Battery Systems J-Tech Solar Training Instructor Biography Jim Parish Jim has been involved in the Solar Industry for over 15 years. He designed and installed the first Photovoltaic
Introduction to Solar Electric Battery Systems J-Tech Solar Training Instructor Biography Jim Parish Jim has been involved in the Solar Industry for over 15 years. He designed and installed the first Photovoltaic
IT 0335 US ARMY INTELLIGENCE CENTER INTRODUCTION TO CELLS AND BATTERIES
 SUBCOURSE IT 0335 EDITION B US ARMY INTELLIGENCE CENTER INTRODUCTION TO CELLS AND BATTERIES INTRODUCTION TO CELLS AND BATTERIES Subcourse Number IT0335 EDITION B US ARMY INTELLIGENCE CENTER FORT HUACHUCA,
SUBCOURSE IT 0335 EDITION B US ARMY INTELLIGENCE CENTER INTRODUCTION TO CELLS AND BATTERIES INTRODUCTION TO CELLS AND BATTERIES Subcourse Number IT0335 EDITION B US ARMY INTELLIGENCE CENTER FORT HUACHUCA,
IT 0335 US ARMY INTELLIGENCE CENTER INTRODUCTION TO CELLS AND BATTERIES
 SUBCOURSE IT 0335 EDITION B US ARMY INTELLIGENCE CENTER INTRODUCTION TO CELLS AND BATTERIES INTRODUCTION TO CELLS AND BATTERIES Subcourse Number IT0335 EDITION B US ARMY INTELLIGENCE CENTER FORT HUACHUCA,
SUBCOURSE IT 0335 EDITION B US ARMY INTELLIGENCE CENTER INTRODUCTION TO CELLS AND BATTERIES INTRODUCTION TO CELLS AND BATTERIES Subcourse Number IT0335 EDITION B US ARMY INTELLIGENCE CENTER FORT HUACHUCA,
Lithium-based Batteries
 Lithium-based Batteries Pioneer work with the lithium battery began in 1912 under G.N. Lewis, but it was not until the early 1970s that the first non-rechargeable lithium batteries became commercially
Lithium-based Batteries Pioneer work with the lithium battery began in 1912 under G.N. Lewis, but it was not until the early 1970s that the first non-rechargeable lithium batteries became commercially
U.S. Department of Energy s Materials Research for Advanced Lithium Ion Batteries
 Page 1 of 6 Page 1 of 6 Return to Web Version U.S. Department of Energy s Materials Research for Advanced Lithium Ion Batteries By: David Howell, Tien Duong, John B. Deppe, Irwin Weinstock, Material Matters
Page 1 of 6 Page 1 of 6 Return to Web Version U.S. Department of Energy s Materials Research for Advanced Lithium Ion Batteries By: David Howell, Tien Duong, John B. Deppe, Irwin Weinstock, Material Matters
From materials to vehicle what, why, and how? From vehicle to materials
 From materials to vehicle what, why, and how? From vehicle to materials Helena Berg Outline 1. Electric vehicles and requirements 2. Battery packs for vehicles 3. Cell selection 4. Material requirements
From materials to vehicle what, why, and how? From vehicle to materials Helena Berg Outline 1. Electric vehicles and requirements 2. Battery packs for vehicles 3. Cell selection 4. Material requirements
Energizer Cylindrical Alkaline Application Manual
 Page 1 of 11 Energizer Cylindrical Alkaline Application Manual Energizer Cylindrical Alkaline (Zn/MnO 2 ) Batteries System Description In answer to a growing need for a high rate source of portable power,
Page 1 of 11 Energizer Cylindrical Alkaline Application Manual Energizer Cylindrical Alkaline (Zn/MnO 2 ) Batteries System Description In answer to a growing need for a high rate source of portable power,
Is there really anything wrong with it? Generation II 2007 Toyota Prius 311,000 miles
 Is there really anything wrong with it? Generation II 2007 Toyota Prius 311,000 miles Always make sure that the HV Disconnect is removed! Always use the proper protective equipment! 1,000 volt gloves Battery
Is there really anything wrong with it? Generation II 2007 Toyota Prius 311,000 miles Always make sure that the HV Disconnect is removed! Always use the proper protective equipment! 1,000 volt gloves Battery
1. Spare Change Flashlight
 . Spare Change Flashlight.. Battery introduction (Adapted from reference 0) Today, batteries are all around us. They power computers, phones, smoke detectors, etc. Batteries are critical not only for current
. Spare Change Flashlight.. Battery introduction (Adapted from reference 0) Today, batteries are all around us. They power computers, phones, smoke detectors, etc. Batteries are critical not only for current
Winter 2016 Conference
 Winter 2016 Conference * Reference: 7x24 International Conference, Spring 2012, Comparison of UPS Alternative Energy Storage Technologies, Syska Hennessy Group, BB&T 3/3/2016 We Will Discuss: What Is A
Winter 2016 Conference * Reference: 7x24 International Conference, Spring 2012, Comparison of UPS Alternative Energy Storage Technologies, Syska Hennessy Group, BB&T 3/3/2016 We Will Discuss: What Is A
COIN CELL CR2477 BRIEF SPECIFICATION
 COIN CELL CR2477 BRIEF SPECIFICATION Model: CR2477 Nominal Voltage: 3.0V Nominal Capacity:1000mAh Standard Discharge with load: 1.5KΩ Weight: 9.5g Stainless steel container ISO9001 Certified UL Certified
COIN CELL CR2477 BRIEF SPECIFICATION Model: CR2477 Nominal Voltage: 3.0V Nominal Capacity:1000mAh Standard Discharge with load: 1.5KΩ Weight: 9.5g Stainless steel container ISO9001 Certified UL Certified
Innovative Uses of Nickel. Joint Study Groups Seminar New & Innovative Applications for Metals. 28 April 2010 Lisbon, Portugal
 Innovative Uses of Nickel Joint Study Groups Seminar New & Innovative Applications for Metals 28 April 2010 Lisbon, Portugal Innovative Uses of Nickel Innovative Projects Incorporate Nickel In transportation
Innovative Uses of Nickel Joint Study Groups Seminar New & Innovative Applications for Metals 28 April 2010 Lisbon, Portugal Innovative Uses of Nickel Innovative Projects Incorporate Nickel In transportation
Electrochemical Power Sources
 Electrochemical Power Sources 1. Reable Batteries A K Shukla and S K Martha A K Shukla is a professor at the Solid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore. His current
Electrochemical Power Sources 1. Reable Batteries A K Shukla and S K Martha A K Shukla is a professor at the Solid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore. His current
AUTOMOTIVE. design engineering. Trends in. New role for carbon Keeping fire at bay, page S14
 Supplement to FEBRUARY 21, 2002 A PENTON PUBLICATION Periodicals USPS 881 Approved Poly Trends in AUTOMOTIVE design engineering New role for carbon Keeping fire at bay, page S14 Tough automotive jobs are
Supplement to FEBRUARY 21, 2002 A PENTON PUBLICATION Periodicals USPS 881 Approved Poly Trends in AUTOMOTIVE design engineering New role for carbon Keeping fire at bay, page S14 Tough automotive jobs are
innovation at work The NanoSafe Battery Alan J. Gotcher, PhD President & CEO Altair Nanotechnologies, Inc. November 29 th, 2006 Research Manufacturing
 Research The NanoSafe Battery Manufacturing Alan J. Gotcher, PhD President & CEO Altair Nanotechnologies, Inc. November 29 th, 2006 Products Partners With the exception of historical information, matters
Research The NanoSafe Battery Manufacturing Alan J. Gotcher, PhD President & CEO Altair Nanotechnologies, Inc. November 29 th, 2006 Products Partners With the exception of historical information, matters
High Power Bipolar Nickel Metal Hydride Battery for Utility Applications
 High Power Bipolar Nickel Metal Hydride Battery for Utility Applications Michael Eskra, Robert Plivelich meskra@electroenergyinc.com, Rplivelich@electroenergyinc.com Electro Energy Inc. 30 Shelter Rock
High Power Bipolar Nickel Metal Hydride Battery for Utility Applications Michael Eskra, Robert Plivelich meskra@electroenergyinc.com, Rplivelich@electroenergyinc.com Electro Energy Inc. 30 Shelter Rock
Energy Storage Technology Roadmap Lithium Ion Technologies
 Energy, Mining and Environment Portfolio Energy Storage Technology Roadmap Lithium Ion Technologies Isobel Davidson, Principal Research Officer 19 November 2014 Energy Storage Technology Roadmap Li ion
Energy, Mining and Environment Portfolio Energy Storage Technology Roadmap Lithium Ion Technologies Isobel Davidson, Principal Research Officer 19 November 2014 Energy Storage Technology Roadmap Li ion
Reliability of Thermal Batteries Melissa Keener
 Reliability of Thermal Batteries Melissa Keener Reliability of Thermal Batteries Thermal batteries are known by different names: molten salt batteries, or liquid sodium batteries. All these refer to the
Reliability of Thermal Batteries Melissa Keener Reliability of Thermal Batteries Thermal batteries are known by different names: molten salt batteries, or liquid sodium batteries. All these refer to the
Performance Loss of Lithium Ion Polymer Batteries Subjected to Overcharge and Overdischarge Abuse
 Naval Research Laboratory Washington, DC 20375-5320 NRL/MR/6110--12-9455 Performance Loss of Lithium Ion Polymer Batteries Subjected to Overcharge and Overdischarge Abuse Corey T. Love Chemical Dynamics
Naval Research Laboratory Washington, DC 20375-5320 NRL/MR/6110--12-9455 Performance Loss of Lithium Ion Polymer Batteries Subjected to Overcharge and Overdischarge Abuse Corey T. Love Chemical Dynamics
New UPS Batteries Keep up so you can keep on backin -up
 #DATACENTERWORLD #CPEXPO CHANNELPARTNERSCONFERENCE.COM DATACENTERWORLD.COM New UPS Batteries Keep up so you can keep on backin -up Dan Lambert Data Center World Certified Vendor Neutral Each presenter
#DATACENTERWORLD #CPEXPO CHANNELPARTNERSCONFERENCE.COM DATACENTERWORLD.COM New UPS Batteries Keep up so you can keep on backin -up Dan Lambert Data Center World Certified Vendor Neutral Each presenter
HIGHLIGHTS. What Every 3M Powered Air Purifying Respirator User Should Know About Batteries
 JobHealth Technical HIGHLIGHTS Information for Occupational Health and Safety Professionals What Every M Powered Air Purifying Respirator User Should Know About Batteries September 006 Vol.. No. 6 Geoff
JobHealth Technical HIGHLIGHTS Information for Occupational Health and Safety Professionals What Every M Powered Air Purifying Respirator User Should Know About Batteries September 006 Vol.. No. 6 Geoff
TRANSPORT OF DANGEROUS GOODS
 Recommendations on the TRANSPORT OF DANGEROUS GOODS Manual of Tests and Criteria Fifth revised edition Amendment 1 UNITED NATIONS SECTION 38 38.3 Amend to read as follows: "38.3 Lithium metal and lithium
Recommendations on the TRANSPORT OF DANGEROUS GOODS Manual of Tests and Criteria Fifth revised edition Amendment 1 UNITED NATIONS SECTION 38 38.3 Amend to read as follows: "38.3 Lithium metal and lithium
Metal-air batteries. Joan Gómez Chabrera Alejandro Andreu Nácher Pablo Bou Pérez
 Metal-air batteries Joan Gómez Chabrera Alejandro Andreu Nácher Pablo Bou Pérez Index 1. Introduction 2. Principle of operation of metal-air batteries 3. Air cathodes 4. Types 5. General aplications 6.
Metal-air batteries Joan Gómez Chabrera Alejandro Andreu Nácher Pablo Bou Pérez Index 1. Introduction 2. Principle of operation of metal-air batteries 3. Air cathodes 4. Types 5. General aplications 6.
Fluorinated Carbon Composite Cathode for a High Energy Lithium Battery
 U.S. Army Research, Development and Engineering Command Fluorinated Carbon Composite Cathode for a High Energy Lithium Battery Inventors: Drs. Shengshui Zhang, Donald Foster, Jeff Wolfenstein, and Jeffrey
U.S. Army Research, Development and Engineering Command Fluorinated Carbon Composite Cathode for a High Energy Lithium Battery Inventors: Drs. Shengshui Zhang, Donald Foster, Jeff Wolfenstein, and Jeffrey
BATTERIES & SUPERCAPS POST MORTEM ANALYSIS PLATFORM EXTERNAL SERVICES
 BATTERIES & SUPERCAPS POST MORTEM ANALYSIS PLATFORM EXTERNAL SERVICES CONTEXT Over the last years a remarkable evolution has taken place by the introduction of new batteries & supercapacitors technologies
BATTERIES & SUPERCAPS POST MORTEM ANALYSIS PLATFORM EXTERNAL SERVICES CONTEXT Over the last years a remarkable evolution has taken place by the introduction of new batteries & supercapacitors technologies
The Discussion of this exercise covers the following points:
 Exercise 1 Battery Fundamentals EXERCISE OBJECTIVE When you have completed this exercise, you will be familiar with various types of lead-acid batteries and their features. DISCUSSION OUTLINE The Discussion
Exercise 1 Battery Fundamentals EXERCISE OBJECTIVE When you have completed this exercise, you will be familiar with various types of lead-acid batteries and their features. DISCUSSION OUTLINE The Discussion
High Energy Rechargeable Li-S Battery Development at Sion Power and BASF
 High Energy Rechargeable Li-S Battery Development at Sion Power and BASF Y. Mikhaylik*, C. Scordilis-Kelley*, M. Safont*, M. Laramie*, R. Schmidt**, H. Schneider**, K. Leitner** *Sion Power Corporation,
High Energy Rechargeable Li-S Battery Development at Sion Power and BASF Y. Mikhaylik*, C. Scordilis-Kelley*, M. Safont*, M. Laramie*, R. Schmidt**, H. Schneider**, K. Leitner** *Sion Power Corporation,
Acme NonStop Power. FNC Cell Technology Sealed fiber nickel-cadmium battery systems For commercial, military and space systems.
 Acme Aerospace Inc., manufactures power supplies and high-performance, sealed FNC batteries for military and commercial aerospace, as well as industrial and satellite/ space applications. Acme NonStop
Acme Aerospace Inc., manufactures power supplies and high-performance, sealed FNC batteries for military and commercial aerospace, as well as industrial and satellite/ space applications. Acme NonStop
Material Safety Data Sheet
 Material Safety Data Sheet 1. Product 1.1 System: Rechargeable Lithium-ion Polymer Battery 2. Composition Information on Components Ingredient CAS Number Percent of Content Classification & Hazard labeling
Material Safety Data Sheet 1. Product 1.1 System: Rechargeable Lithium-ion Polymer Battery 2. Composition Information on Components Ingredient CAS Number Percent of Content Classification & Hazard labeling
Safeguarding lithium-ion battery cell separators
 Safeguarding lithium-ion battery cell separators Executive Summary Technical advances in the design and construction of lithium-ion battery cells have played an essential role in the widespread deployment
Safeguarding lithium-ion battery cell separators Executive Summary Technical advances in the design and construction of lithium-ion battery cells have played an essential role in the widespread deployment
Battery Market Trends and Safety Aspects
 Battery Market Trends and Safety Aspects Adam Sobkowiak PhD, Battery Technologies adam.sobkowiak@etteplan.com 2018-01-17, Breakfast Seminar at Celltech, Kista 1 Battery Market Trends Engineering with a
Battery Market Trends and Safety Aspects Adam Sobkowiak PhD, Battery Technologies adam.sobkowiak@etteplan.com 2018-01-17, Breakfast Seminar at Celltech, Kista 1 Battery Market Trends Engineering with a
Acme NonStop Power. FNC Cell Technology
 Acme NonStop Power................................................................................................................ FNC Cell Technology Sealed fiber nickel-cadmium battery systems For commercial,
Acme NonStop Power................................................................................................................ FNC Cell Technology Sealed fiber nickel-cadmium battery systems For commercial,
Study of Thermal and Electrochemical Characteristics of Li-ion Battery
 Study of Thermal and Electrochemical Characteristics of Li-ion Battery 1 Anand R. Savandkar, 2 D. S. Watvisave 1 P.G. Student, 2 Assistant Professor (Dept. of Mechanical Engineering, SCoE, Pune University,
Study of Thermal and Electrochemical Characteristics of Li-ion Battery 1 Anand R. Savandkar, 2 D. S. Watvisave 1 P.G. Student, 2 Assistant Professor (Dept. of Mechanical Engineering, SCoE, Pune University,
Rechargeable Batteries
 Nanomaterial approaches to enhance lithium ion batteries Potential Environmental Benefits of Nanotechnology: Fostering Safe Innovation-Led Growth July 17 th, 2009 Brian J. Landi Assistant Professor of
Nanomaterial approaches to enhance lithium ion batteries Potential Environmental Benefits of Nanotechnology: Fostering Safe Innovation-Led Growth July 17 th, 2009 Brian J. Landi Assistant Professor of
Zinc-Air Batteries for UAVs and MAVs
 Zinc-Air Batteries for UAVs and MAVs Dr. Neal Naimer, Vice President R&D (speaker) Binyamin Koretz, Vice President Business Development Ronald Putt, Director of Technology Electric Fuel Corporation Auburn,
Zinc-Air Batteries for UAVs and MAVs Dr. Neal Naimer, Vice President R&D (speaker) Binyamin Koretz, Vice President Business Development Ronald Putt, Director of Technology Electric Fuel Corporation Auburn,
Battery Selection, Safety, and Monitoring in Mobile Applications
 Battery Selection, Safety, and Monitoring in Mobile Applications Yevgen Barsukov, Texas Instruments ABSTRACT The battery is often considered by engineers as a constant voltage source that does not require
Battery Selection, Safety, and Monitoring in Mobile Applications Yevgen Barsukov, Texas Instruments ABSTRACT The battery is often considered by engineers as a constant voltage source that does not require
Li-Ion battery Model. Octavio Salazar. Octavio Salazar
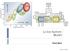 Li-Ion battery Model 1 Energy Storage- Lithium Ion Batteries C-PCS: Control and Power Conditioning System Energy Storage- Lithium Ion Batteries Nature [0028-0836] Tarascon (2001) volume: 414 issue: 6861
Li-Ion battery Model 1 Energy Storage- Lithium Ion Batteries C-PCS: Control and Power Conditioning System Energy Storage- Lithium Ion Batteries Nature [0028-0836] Tarascon (2001) volume: 414 issue: 6861
Battery nomenclature From Wikipedia, the free encyclopedia
 Page 1 of 13 Battery nomenclature From Wikipedia, the free encyclopedia Standard battery nomenclature describes portable dry cell batteries that have physical dimensions and electrical characteristics
Page 1 of 13 Battery nomenclature From Wikipedia, the free encyclopedia Standard battery nomenclature describes portable dry cell batteries that have physical dimensions and electrical characteristics
FRAUNHOFER INSTITUTE FOR CHEMICAL TECHNOLOGY ICT REDOX-FLOW BATTERY
 FRAUNHOFER INSTITUTE FOR CHEMICAL TECHNOLOGY ICT REDOX-FLOW BATTERY REDOX-FLOW BATTERY REDOX-FLOW BATTERY Redox-flow batteries are efficient and have a longer service life than conventional batteries.
FRAUNHOFER INSTITUTE FOR CHEMICAL TECHNOLOGY ICT REDOX-FLOW BATTERY REDOX-FLOW BATTERY REDOX-FLOW BATTERY Redox-flow batteries are efficient and have a longer service life than conventional batteries.
Ionic Additives for Electrochemical Devices Using Intercalation Electrodes
 U.S. Army Research, Development and Engineering Command Ionic Additives for Electrochemical Devices Using Intercalation Electrodes Inventor: Dr. Kang Xu ARL 09-18 February 16, 2011 Technology Overview
U.S. Army Research, Development and Engineering Command Ionic Additives for Electrochemical Devices Using Intercalation Electrodes Inventor: Dr. Kang Xu ARL 09-18 February 16, 2011 Technology Overview
Implementation and development of standards for Lithium-ion energy storage technologies within the South African context
 Implementation and development of standards for Lithium-ion energy storage technologies within the South African context by Nico Rust, Nelson Mandela University uyilo EMTIP uyilo emobility Technology Innovation
Implementation and development of standards for Lithium-ion energy storage technologies within the South African context by Nico Rust, Nelson Mandela University uyilo EMTIP uyilo emobility Technology Innovation
