D aniell cell, invented by the British chemist John Frederic Daniell in 1836, is popularly known as a kind of
|
|
|
- August Roderick Campbell
- 6 years ago
- Views:
Transcription
1 OPEN SUBJECT AREAS: ELECTROCHEMISTRY BATTERIES Received 6 September 2014 Accepted 15 October 2014 Published 5 November 2014 Correspondence and requests for materials should be addressed to Y.W. (ygwang@ fudan.edu.cn) Re-building Daniell Cell with a Li-ion exchange Film Xiaoli Dong, Yonggang Wang & Yongyao Xia Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, Fudan University, Shanghai , China. Daniell cell (i.e. Zn-Cu battery) is widely used in chemistry curricula to illustrate how batteries work, although it has been supplanted in the late 19th century by more modern battery designs because of Cu -crossover-induced self-discharge and un-rechargeable characteristic. Herein, it is re-built by using a ceramic Li-ion exchange film to separate Cu and Zn electrodes for preventing Cu -crossover between two electrodes. The re-built Zn-Cu battery can be cycled for 150 times without capacity attenuation and self-discharge, and displays a theoretical energy density of 68.3 Wh kg. It is more important that both electrodes of the battery are renewable, reusable, low toxicity and environmentally friendly. Owing to these advantages mentioned above, the re-built Daniell cell can be considered as a promising and green stationary power source for large-scale energy storage. D aniell cell, invented by the British chemist John Frederic Daniell in 1836, is popularly known as a kind of zinc-copper battery which takes advantage of a porous barrier between the two metals 1,2. Once used widely in the European telegraph industry, it was supplanted in the late 19th century by more modern battery designs. Today, it is primarily used in the chemistry curricula to demonstrate how batteries work 1,2. As shown in our chemistry curricula, the typical Daniell cell works with a salt bridge connecting the anode electrode of a zinc sulfate solution and an immersed zinc plate as well as the cathode electrode of a copper sulfate solution and an immersed copper plate (Figure S1). When discharged, the anode of zinc will be oxidized according to the equation 1: Zn (s)?zn 2z (aq) z2e{ ð1þ At the same time, the cathode of copper ions will be reduced following the equation 2: Cu 2z (aq) z2e{?cu (s) ð2þ Therefore, we can see the total reaction as [Zn(s)] 1 [Cu (aq)] R [Zn (aq)] 1 [Cu(s)] during the discharge course. These two processes cause copper solid to accumulate at the cathode and the zinc electrode to dissolve into the solution and show a theoretical potential of 1.1 V at 25uC. Without the salt bridge, the reaction will occur directly (i.e. Cu will be deposited on Zn anode) and the electron flow will not be directed through the outer wire to supply power for use. To be exactly, the cations in the salt bridge migrate to the container containing the copper electrode to replace the copper ions being consumed, while the anions in the salt bridge migrate toward the zinc side, where they keep the solution containing the newly formed zinc cations electrically neutral. However, it is a pity to realize that the salt bridge (or porous barrier) can only alleviate the Cu crossover. At the open circuit condition, Cu still can slowly diffuse from the Cu electrode room to Zn electrode room through the salt bridge (or porous barrier), and then the Cu is reduced into metallic Cu on the surface of Zn electrode, indicating a serious self-discharge and/or suicide process (see Figure S2 for detail). It is also undoubted that Daniell Cell is not rechargeable, because recharge would much aggravate the Cu crossover, indicating a battery-killing process (see Figure S3 for detail). Owing to the serious self-discharge and the unrechargeable characteristic, Daniell cell was supplanted by more modern rechargeable battery technologies, such as Lead-acid battery developed in 1859, Ni-Cd (Nickel/ Cadmium) battery developed in 1909, Ni-MH (Nickel/Metal hydride) battery developed in 1975 and Li-ion battery developed in Up to present, these aqueous-electrolyte-based rechargeable batteries (i.e. Lead-acid, Ni-Cd and Ni-MH, etc.) and the organic-electrolyte-based Li-ion batteries are still used for diverse range of applications 3,4. Due to their higher energy density and cycling stability, the Li-ion batteries using two intercalated compounds (e.g. carbon anode and LiCoO 2 cathode) in an organic solution electrolyte occupy the main market of SCIENTIFIC REPORTS 4 : 6916 DOI: /srep
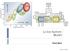
2 battery, and are widely used for various electronic devices, from portable devices (such as cellular phones, notebook-size personal computers, and so on) to electric vehicles (EVs) 3,4. Owing to the accelerated energy consumption and aggravated global warming, it is undoubted that future economy must be based on green and sustainable energy source, such as solar energy, wind energy, etc. As a result, utilization of these sustainable energy sources has been a hot topic. It is well known that the efficient utilization of these sustainable energy sources depends on large-scale stationary energy storage batteries. However, the organic-electrolyte-based Liion battery is difficult to play the role of large-scale energy storage device for these sustainable energy sources. Despite the remarkable performance of these organic based systems mentioned above, they suffer from the use of highly toxic and flammable solvents, which can cause safety hazards if used improperly, such as overcharging or short-circuiting 3,4. Especially, numerous lithium-ion battery accidents causing fires and explosions have been reported. As a response, these high safety aqueous rechargeable batteries should be the promising candidates for large-scale energy storage, although they display lower energy density. Unfortunately, the application of these commercialized aqueous rechargeable batteries (i.e. Lead-acid, Ni-Cd and Ni-MH, etc.) in large-scale energy storage may be held back by the toxicity of electrode materials (such as Pb and Cd) and/or limited storage of raw materials for electrode (such as Metal hydride). Therefore, aqueous electrolyte Li-ion batteries arrest much attention in recent years, despite their poor cycling stability 5,6. However, the raw materials for intercalation compounds (i.e. electrode materials for Li-ion batteries) are not sustainable because cobalt, nickel, manganese and lithium must be natural resources. In addition, most intercalation compounds for Li-ion batteries are prepared by hightemperature solid-state reaction, which also results in consumption of energy and CO 2 emission 3. As we know, the carbon footprint of organic Li-ion batteries even reaches 70 Kg CO 2 per kwh 3. Especially, the reuse of these intercalation compounds in the used batteries is very difficult and complex. As a response, it may be the time for us to reconsider Daniell cell as a candidate for large-scale energy storage, because both Zn and Cu are low toxicity, renewable and reusable. However, the precondition is to well deal with the problem from Cu crossover on operating process, which obviously is a great challenge. Herein a ceramic lithium super-ionic conductor (LATSP, Li 11x1y Al x Ti 2-x Si y P 3-y O 12 ) thin film, which is recently used in aqueous/non-aqueous double-electrolyte batteries 7 23, was used to separate the Cu cathode and Zn anode to build a stable and rechargeable Zn-Cu battery. The resulting Zn-Cu battery can be cycled for 150 times without obvious capacity attenuation, and the open circuit voltage investigation shows that the potential can keep stable for over 100 h without any loss of the capacity. Results The structure and operating mechanism of the rechargeable Zn-Cu battery are illustrated in Figure 1, where it can be detected that a Cu cathode (1 cm 2 ) in 0.25 ml 2 M LiNO 3 electrolyte solution and a Zn anode (1 cm 2 ) in 0.25 ml 1 M Zn(NO 3 ) 2 electrolyte solution are separated by a ceramic LATSP film. Detailed information about the as-prepared rechargeable Zn-Cu battery is given in Figure S4. On charge, metallic Cu (i.e. cathode) is oxidized into Cu and the Zn in anodic room is reduced into metallic Zn at the surface of anode, while Li ions in the cathodic room diffuse to the anodic room through the LATSP ceramic film to balance the charges. Simultaneously, electrons are transferred from Cu cathode to Zn anode through the outer circuit. Discharge reverses the charge process, and is similar with conventional Daniell cell. As a result, the total reaction within the developed rechargeable Zn-Cu battery can be summarized as: Figure 1 Schematic illustration and operating mechanism of rechargeable Zn-Cu battery with a Li-ion exchange membrane. ½Cuz2LiNO 3 ŠzZn(NO 3 ) 2 /?Cu(NO 3 ) 2 z½2lino 3 zznš Figure 2 gives the cyclic profile of the rechargeable Zn-Cu battery with an applied current of 0.25 ma. In this investigation, a battery is charged for 6 hours to reach a charge capacity of 1.5 mah, and then the battery is discharged to 0.2 V. As shown in Figure 2a, the battery displays a flat charge voltage of 1.25 V for 6 hours and a flat discharge voltage of about 0.8 V for about 6 hours with the applied current of 0.25 ma. About 1.78 mg metallic Cu is transformed into Cu in cathodic room through the 6 h charge with a current of 0.25 ma, and simultaneously Zn in the electrolyte of anodic room is reduced into metallic Zn. At the same time, Li-ions diffuse from cathodic room to anodic room to balance the charge. In order to further clarify the coulombic efficiency, we calculated the capacity (mah g Cu ) based on the mass of consumed metallic Cu (or generated Cu ) over charge process. As shown in Figure 2b, the achieved discharge capacity of 843 mah g Cu is equal to the charge capacity at initial cycle. However, the discharge capacity at 20th, 75th and 150th cycle is slightly smaller than corresponding charge capacity, indicating that the Coulombic efficiency of the Zn-Cu battery is only close to 100%. In other words, there is no obvious crossover of Cu over charge-discharge process. Especially, the battery almost keeps the constant discharge voltage and capacity at various cycles, suggesting perfect cyclic ability (Figure 2a and 2b). The rechargeable characteristic should be attributed to that the ceramic Li-ion exchange film (i.e. LTASP) can efficiently prevent the Cu crossover, which is confirmed by electrochemical impedance spectroscopic (EIS) investigation (Figure S5). Furthermore, it also should be noted that there is a clear degradation of the charge voltage curved at the beginning of the charging step. This phenomenon may be ascribed to the formation of Cu 2 O and the growth of dendrite on the surface of cathode, and need further investigation (see supplementary information for detailed discussion). Herein, we also employed a proton exchange membrane (Nafion 117) to separate the Cu cathode and Zn anode, and investigated its cyclic performance at the same experiment condition for comparison. Unfortunately, being similar to the saltbridge-based Daniell cell (Figure S3), the Nafion-film-based Zn-Cu battery can not be recharged (Figure S6). Although the proton exchange membrane permits the fast pass of Li 1, it also permits the pass of Cu (See Figure S7 and corresponding discussion). In order to further clarify the stability of the rechargeable Zn-Cu battery with a LTASP separator, self-discharge investigation of the battery was conducted. In this measurement, the battery was first charged for 10 hours with an applied current density of 0.1 ma. Then, the ð3þ SCIENTIFIC REPORTS 4 : 6916 DOI: /srep
3 Figure 2 Cyclic profile of the rechargeable Zn-Cu battery. (a) Cell voltage vs. time. (b) Cell voltage vs. capacity. [In this investigation, a battery is charged for 6 hours to reach a charge capacity of 1.5 mah, and then the battery is discharged to 0.2 V with an applied current of 0.25 ma.] charged battery was kept at open circuit voltage condition (OCV) for 100 hours (Figure 3a). Finally, the resulting battery was discharged with a current of 0.1 ma (Figure 3a). As shown in Figure 3b, the battery displays a flat discharge voltage for about 10 hours at the current of 0.1 ma (i.e. a discharge capacity of around 843 mah g Cu ), indicating that the generated Cu has been converted into metallic Cu through the electrochemical reduction on discharge process. Herein, we also carried out a control study as a comparison where a Zn-Cu battery was built in the same way as the one described for Figure 3a, and was discharged without OCV storage at the same current of 0.1 ma. In this measurement, the Zn-Cu battery was first charged for 10 hours with an applied current density of 0.1 ma, and then was discharged with a current of 0.1 ma without OCV storage. Discharge curve of the Zn-Cu battery without OCV storage is given in Figure 3c, where it can be observed that the battery displays a flat discharge voltage for about 10 hours, indicating a capacity of around 843 mah g Cu. The comparison between Figure 3b and 3c demonstrates that the conventional Daniell cell can be developed as a rechargeable Zn-Cu battery without self-discharge. In addition, a small voltage plateau arising from the hydrogen evolution can be observed at the end of the discharge, indicating the charge/discharge efficiency does not reach 100%. Figure 4a gives the rate performance of the rechargeable Zn-Cu battery using a LATSP separator. In this investigation, the battery was charged for 6 h with a current of 1 ma, and then discharged with different currents. The capacities at different currents are also just calculated based on the mass of consumed Cu (or generated Cu ) over charge process. As shown in Figure 4a, the polarization increases obviously with the growth of discharge currents, suggesting a large internal resistance. Obviously, the low conductivity of LATSP will much limit the power output of this kind of LATSP-based Zn-Cu battery. However, this drawback may be solved by adjusting the operating model of this kind of battery. It is well known that proton exchange membrane (i.e. Nafion film) is of high ionic conductivity. For instance, we also investigated the rate performance of Nafionbased Zn-Cu battery at the same condition. In this investigation, a LATSP-based Zn-Cu battery was first charged for 6 hours to form Cu solution in the cathodic room. Then, the generated Cu solution is removed to the cathodic room of a Nafion-based Zn-Cu battery (Zn/Nafion/Cu) for discharge measurement. Figure 4b gives the discharge profile of the Nafion-based Zn-Cu battery at different currents. It can be observed from Figure 4b that the Nafion-based Zn-Cu battery displays higher operating voltage even at much higher discharge currents, compared with that of LATSP-based Zn-Cu battery. However, it should be noted that the discharge capacity is lower than the theoretical capacity (i.e. 843 mah g Cu ) of metallic Cu (or Cu ), indicating a small amount of Cu crossover over discharge. Furthermore, it can be observed that an additional voltage plateau (about 0.4 V) appears at the end of discharge of Nafion-based Zn-Cu battery, which should be attributable to the H 2 evolution over discharge process. According to the operating mechanism of the rechargeable Zn-Cu battery (see Figure 1), Li ions should diffuse from anodic room to cathodic room to balance the charge over discharge process. However, the Nafion film also permits the fast pass of H 1. As a result, the protons also diffuse from anodic room to cathodic room in parallel with the Li ions diffusion on discharge. The proton crossover improves the H 1 concentration in the cathodic room, and thus enhances the H 2 evolution potential over discharge SCIENTIFIC REPORTS 4 : 6916 DOI: /srep
4 Figure 3 Self-discharge investigation of the rechargeable Zn-Cu battery. (a) OCV and discharge curves. (b) Enlargement of discharge curve after OCV test. (c) Discharge curve without OCV storage. process. The results from Figure 4a and 4b suggest that we can improve the power output of rechargeable Zn-Cu battery through adjusting operating method. As shown in Figure 4c, the LATSPbased Zn-Cu battery can be used for charge storage (i.e. to form Cu solution and metallic Zn through charge process), and then the charge products (e.g. Cu ) can be flowed to the Nafion-based Zn-Cu battery for high rate discharge when high power output is needed. This technology may be similar with flow batteries. In order to further evaluate the power output of the rechargeable Zn-Cu battery, higher concentration Cu(NO 3 ) 2 solution (1 M) was employed as cathodic electrolyte to investigate the power performance of Nafion-based Zn-Cu battery and LATSP-based Zn-Cu battery, respectively. As shown in Figure 5, the discharge voltage of Nafion-based Zn-Cu battery is still higher than 0.5 V even at the high current of 35 ma (i.e. 35 ma cm 22 ). However, the discharge voltage of LATSP-based Zn-Cu battery reduces to 0.5 V at the current of 3 ma (see inset of Figure 5). The result from Figure 5 further confirms that Nafion-based Zn-Cu battery can efficiently offset the low power characteristic of LATSP-based Zn-Cu battery. Discussion Above results have demonstrated that the new type Zn-Cu battery is of perfect cyclic ability and high stability, and its power output can also be improved through proper operating method. As a result, the next logic step is to evaluate its theoretical energy density. It should be noted that the above capacity (i.e. mah g Cu ) calculated from the consumed Cu or Cu is just used to describe the reversibility of battery, and thus can not be employed to evaluate the true energy density of the battery. According to Equation 3, the active materials of the new type Zn-Cu battery include Cu, LiNO 3 and Zn(NO 3 ) 2. Furthermore, the solubility in water of LiNO 3 and Zn(NO 3 ) 2 should also be considered in the calculation of energy density. Accordingly, the theoretical energy density of the rechargeable Zn-Cu battery based on the total weight of the Cu cathode, the LiNO 3 electrolyte and the aqueous Zn(NO 3 ) 2 anode can be calculated from equation (4) Q m cu V Q m cu V E~ ~ m cathode zm electrolyte zm anode m Cu z(m LiNO3 zm 1 H2O)z(m Zn(NO3) 2 zm 2 H2O) ð4þ where E is the energy density (Wh kg ), V is the discharge voltage (,0.8 V), Q is the theoretical capacity of Cu (843 mah g Cu ), m cu is the mass of Cu cathode (63.55 g; 1 mol), m LiNO3 is the mass of LiNO 3 in electrolyte ( g; 2 mol), and m Zn(NO3)2 is the mass of Zn(NO 3 ) 2 in aqueous anode ( g; 1 mol). The m 1 H2O and m 2 H2O are the masses of water for LiNO 3 dissolution (100 g H 2 O) and Zn(NO 3 ) 2 dissolution (137 g H 2 O), which are calculated based on their solubility at 30uC. According to equation (4), the calculated energy density can reach 68.3 Wh kg. It should be noted that 2 M SCIENTIFIC REPORTS 4 : 6916 DOI: /srep
5 Figure 4 Discharge curves of Zn-Cu batteries at different currents. (a) Discharge curves of LATSP-based Zn-Cu battery. (b) Discharge curves of Nafion-based Zn-Cu battery. (c) Schematic illustrating the combination application between LATSP-based Zn-Cu battery and Nafion-based Zn-Cu battery. LiNO 3 solution and 1 M Zn(NO 3 ) 2 solution were employed in our experiment to demonstrate the performance of rechargeable Zn-Cu battery. However, the calculation of theoretical energy of the rechargeable Zn-Cu battery is based on the solubility of LiNO 3 and Zn(NO 3 ) 2 at 30uC. Typically, the electrode/electrolyte material weighs about 50% of the total weight of the large-size practical battery. Thus, the practical specific energy of the new type Zn-Cu battery Figure 5 Rate performance (discharge voltage vs. current) of Nafionbased Zn-Cu battery investigated with 1 M Cu(NO 3 ) 2 solution. The inset is the discharge voltage vs. current of LATSP-based Zn-Cu battery tested at the same condition. near 34 Wh kg can be expected, which is compatible with commercialized lead acid battery (30, 40 Wh kg ) 3,24 and flow battery (25 30 Wh kg ) 3,24. It is more important that the electrode reactions of the battery depend on the dissolution deposition of metallic Cu and Zn, indicating that Cu-cathode and Zn-anode are renewable and reusable. For example, the Cu-cathode or Zn-anode in a used rechargeable Zn Cu battery can be directly recycled to fabricate a new electrode. However, for present commercialized aqueous battery (e.g. lead acid, Ni-Cd and Ni-MH batteries), the recycle of electrode materials is of a great challenge. Especially, both electrode material and electrolyte of rechargeable Zn-Cu battery are low toxicity, which is quite important for low carbon society. Therefore, the rechargeable Zn-Cu battery can be considered as a promising power source for large-scale energy storage. On the other hand, the charge rate (e.g. energy storage rate) of rechargeable Zn-Cu battery is still much limited by the low conductivity of the ceramic LATSP film, and can not be offset by the above Nafion-based Zn-Cu battery. As a response, this kind of Zn-Cu battery may be only suitable for solar energy storage at present stage. In the further investigation, it is necessary to improve the conductivity of ion-exchange membrane and enhance the operating temperature of the battery. Especially, enhancing the operating temperature can not only improve the conductivity of the ceramic ion-exchange membrane but also increase the solubility of active materials, which thus can improve both energy and power density of the system. It should also be noted that dendrite growth inevitably occurs on the Cu and Zn electrode over cycling process. Although the short circuit hazards in the rechargeable Zn- Cu battery can be eliminated because the rigid ceramic LATSP film is hardly pierced by the dendrites on electrode, the dendrite growth still could limit the efficient utilization of Cu/Zn electrode over long-time cycling. Therefore, more researches should be done to solve the SCIENTIFIC REPORTS 4 : 6916 DOI: /srep
6 problem, for example, some kind of electrolyte additive might be useful to produce smooth coating during the electrodeposition. In summary, the old Daniell cell was re-built as a stable and rechargeable battery through the Li-ion exchange film that can efficiently prevent the crossover of Cu. The theoretical energy density of the new type Zn-Cu battery can reach 68.3 Wh kg which is compatible with current aqueous rechargeable batteries. It is more important that both electrodes of the battery are renewable, reusable, low toxicity and environmentally friendly. It can be expected that this investigation not only gives a new birth for the very old Daniell cell, but also provides a new promising and green power source for largescale energy storage. Method Materials and Preparation of Zn-Cu battery. The reagents [e.g. Zn plate, Cu plate, Zn(NO 3 ) 2 and LiNO 3 ] were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai). Zinc plate (0.2 mm in thickness, 99.9%) and copper plate (0.1 mm in thickness, 99.9%) were polished before battery assembly. The cm 2 ceramic lithium super-ionic conductor film (LATSP, Li 11x1y Al x Ti 2-x Si y P 3-y O 12 purchased from Ohara Inc., Japan) with a thickness of 0.15 mm and a Li-ion conductivity of Scm was used as received. The glass microfiber filter was obtained from WhatmanH Anodisc Inorganic Membranes. 2 M LiNO 3 solution and 1 M Zn(NO 3 ) 2 solution were used as cathodic electrolyte and anodic electrolyte, respectively. Detailed information about the battery assembly was given in Figure S4. Electrochemical Characterization. Electrochemical tests were performed on HOKUTO DENKO battery charge/discharge system HJ series (Japan) controlled by a computer. The electrochemical impedance spectroscopy (EIS) was carried out on Solartron Instrument Model 1287 in the frequency range of Hz with the AC signal amplitude of 10 mv. 1. Boulabiar, A., Bouraoui, K., Chastrette, M. & Abderrabba, M. A historical analysis of the Daniell cell and electrochemistry teaching in French and Tunisian textbooks. J. Chem. Educ. 81, (2004). 2. Martins, G. F. Why the Daniell cell works. J. Chem. Educ. 67, (1990). 3. Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, (2008). 4. Bruce, P. G., Scrosati, B. & Tarascon, J. M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 47, (2008). 5. Luo, J. Y., Cui, W. J., He, P. & Xia, Y. Y. Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat. Chem. 2, (2010). 6. Wang, Y. G., Yi, J. & Xia, Y. Y. Recent progress in aqueous lithium-ion batteries. Adv. Energy Mater. 2, (2012). 7. Li, L. J., Zhao, X. S. & Manthiram, A. A dual-electrolyte rechargeable Li-air battery with phosphate buffer catholyte. Electrochem. Commun. 14, (2012). 8. Li, L. J., Zhao, X. S., Fu, Y. Z. & Manthiram, A. Polyprotic acid catholyte for high capacity dual-electrolyte Li air batteries. Phys. Chem. Chem. Phys. 14, (2012). 9. Lu, Y. H. & Goodenough, J. B. Rechargeable alkali-ion cathode-flow battery. J. Mater. Chem., (2011). 10. Lu, Y. H., Goodenough, J. B. & Kim, Y. Aqueous cathode for next-generation alkali-ion batteries. J. Am. Chem. Soc. 133, (2011). 11. Zhang, T. et al. A novel high energy density rechargeable lithium/air battery. Chem. Commun. 46, (2010). 12. Zhang, T. et al. Li/polymer electrolyte/water stable lithium-conducting glass ceramics composite for lithium air secondary batteries with an aqueous electrolyte. J. Electrochem. Soc. 155, A965 A969 (2008). 13. Zhao, Y., Wang, L. N. & Byon, H. R. High-performance rechargeable lithiumiodine batteries using triiodide/iodide redox couples in an aqueous cathode. Nat. Commun. 4, (2013). 14. Zhao, Y. & Byon, H. R. High-performance lithium-iodine flow battery. Adv. Energy Mater. 3, (2013). 15. Zhao, Y. et al. A reversible Br 2 /Br - redox couple in the aqueous phase as a highperformance catholyte for alkali-ion batteries. Energy Environ. Sci. 7, (2014). 16. Zhou, H. S., Wang, Y. G. Li, H. Q. & He, P. The development of a new type of rechargeable batteries based on hybrid electrolytes. ChemSusChem. 3, (2010). 17. He, P., Wang, Y. G. & Zhou, H. S. A Li-air fuel cell with recycle aqueous electrolyte for improved stability. Electrochem. Commun. 12, (2010). 18. Wang, Y. G. & Zhou, H. S. A lithium-air battery with a potential to continuously reduce O2from air for delivering energy. J. Power Sources. 195, (2010). 19. Wang, Y. G. & Zhou, H. S. A lithium air fuel cell using copper to catalyze oxygenreduction based on copper-corrosion mechanism. Chem. Commun. 46, (2010). 20. Wang, Y. G. & Zhou, H. S. A new type rechargeable lithium battery based on a Cucathode. Electrochem. Commun. 11, (2009).. Wang, Y. G., He, P. & Zhou, H. S. A lithium air capacitor battery based on a hybrid electrolyte. Energy Environ. Sci. 4, (2011). 22. Wang, Y. R., He, P. & Zhou, H. S. Li-redox flow batteries based on hybrid electrolytes: at the cross road between Li-ion and redox flow batteries. Adv. Energy Mater. 2, (2012). 23. Lim, H. D. et al. Superior rechargeability and efficiency of lithium oxygen batteries:hierarchical air electrode architecture combined with a soluble catalyst. Angew. Chem. Int. Ed. 53, (2014). 24. Dunn, B., Kamath, H. & Tarascon, J. M. Electrical energy storage for the grid: a battery of choices. Science 334, (2011). Acknowledgments The authors acknowledge funding support from the Natural Science Foundation of China (333002, ), the State Key Basic Research Program of PRC (2013CB934103, 2014CB932301), Shanghai Pujiang Program (13PJ ), and Shanghai Science & Technology Committee (11DZ , 08DZ ). Author contributions X.L.D. and Y.G.W. designed the experiments and discussed the interpretation of results. X.L.D., Y.G.W. and Y.Y.X. discussed the results, co-wrote the paper and participated in the manuscript revision. Additional information Supplementary information accompanies this paper at scientificreports Competing financial interests: The authors declare no competing financial interests. How to cite this article: Dong, X., Wang, Y. & Xia, Y. Re-building Daniell Cell with a Li-ion exchange Film. Sci. Rep. 4, 6916; DOI: /srep06916 (2014). This work is licensed under a Creative Commons Attribution-NonCommercial- ShareAlike 4.0International License. The images or other third party material in this article are included in the article s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit creativecommons.org/licenses/by-nc-sa/4.0/ SCIENTIFIC REPORTS 4 : 6916 DOI: /srep
Storage: the state of the technology
 Storage: the state of the technology Torbjörn Gustafsson Ångström Advanced Battery Centre Department of Materials Chemistry Uppsala University 1 Acknowledgements Ångström Advanced Battery Centre 2 Over
Storage: the state of the technology Torbjörn Gustafsson Ångström Advanced Battery Centre Department of Materials Chemistry Uppsala University 1 Acknowledgements Ångström Advanced Battery Centre 2 Over
There are several technological options to fulfill the storage requirements. We cannot use capacitors because of their very poor energy density.
 ET3034TUx - 7.5.1 - Batteries 1 - Introduction Welcome back. In this block I shall discuss a vital component of not only PV systems but also renewable energy systems in general. As we discussed in the
ET3034TUx - 7.5.1 - Batteries 1 - Introduction Welcome back. In this block I shall discuss a vital component of not only PV systems but also renewable energy systems in general. As we discussed in the
THE BUSINESS CASE FOR INDUSTRIAL-SCALE BATTERIES
 11 THE BUSINESS CASE FOR INDUSTRIAL-SCALE BATTERIES TECHNOLOGY OVERVIEW Batteries store electricity as chemical energy so that it can be recovered for later use. There are many different battery types;
11 THE BUSINESS CASE FOR INDUSTRIAL-SCALE BATTERIES TECHNOLOGY OVERVIEW Batteries store electricity as chemical energy so that it can be recovered for later use. There are many different battery types;
Metal-air batteries. Joan Gómez Chabrera Alejandro Andreu Nácher Pablo Bou Pérez
 Metal-air batteries Joan Gómez Chabrera Alejandro Andreu Nácher Pablo Bou Pérez Index 1. Introduction 2. Principle of operation of metal-air batteries 3. Air cathodes 4. Types 5. General aplications 6.
Metal-air batteries Joan Gómez Chabrera Alejandro Andreu Nácher Pablo Bou Pérez Index 1. Introduction 2. Principle of operation of metal-air batteries 3. Air cathodes 4. Types 5. General aplications 6.
Development of battery materials with world s highest performance
 Tokyo University of Agriculture and Technology Nippon Chemi-Con Corporation May 6, 2010 Applying nano-hybrid technology to the next generation lithium-ion battery Development of battery materials with
Tokyo University of Agriculture and Technology Nippon Chemi-Con Corporation May 6, 2010 Applying nano-hybrid technology to the next generation lithium-ion battery Development of battery materials with
Use of Aqueous Double Layer Ultracapacitor using Hybrid CDI-ED Technology for the use in Hybrid Battery Systems
 Use of Aqueous Double Layer Ultracapacitor using Hybrid CDI-ED Technology for the use in Hybrid Battery Systems Overview By Robert Atlas, Aqua EWP,LLC. September 2007 Aqua EWP. has for the last 10 years
Use of Aqueous Double Layer Ultracapacitor using Hybrid CDI-ED Technology for the use in Hybrid Battery Systems Overview By Robert Atlas, Aqua EWP,LLC. September 2007 Aqua EWP. has for the last 10 years
Lithium Ion Batteries - for vehicles and other applications
 Lithium Ion Batteries - for vehicles and other applications Tekes 2008-12-03 Kai Vuorilehto / European Batteries What do we need? High energy (Wh/kg) driving a car for 5 hours High power (W/kg) accelerating
Lithium Ion Batteries - for vehicles and other applications Tekes 2008-12-03 Kai Vuorilehto / European Batteries What do we need? High energy (Wh/kg) driving a car for 5 hours High power (W/kg) accelerating
Battery Power for All-Electric Road Vehicles John B. Goodenough and M. Helena Braga The University of Texas at Austin, and of Porto, Portugal
 Battery Power for All-Electric Road Vehicles John B. Goodenough and M. Helena Braga The University of Texas at Austin, and of Porto, Portugal Modern Society runs on the energy stored in fossil fuels. This
Battery Power for All-Electric Road Vehicles John B. Goodenough and M. Helena Braga The University of Texas at Austin, and of Porto, Portugal Modern Society runs on the energy stored in fossil fuels. This
Energy Storage (Battery) Systems
 Energy Storage (Battery) Systems Overview of performance metrics Introduction to Li Ion battery cell technology Electrochemistry Fabrication Battery cell electrical circuit model Battery systems: construction
Energy Storage (Battery) Systems Overview of performance metrics Introduction to Li Ion battery cell technology Electrochemistry Fabrication Battery cell electrical circuit model Battery systems: construction
Portable Power & Storage
 Portable Power & Storage NMTC Disruptive Technology Summit and TECH CONN3CT Workshops 28 April 2017 Edward J. Plichta Chief Scientist for Power & Energy Command Power & Integration Directorate Aberdeen
Portable Power & Storage NMTC Disruptive Technology Summit and TECH CONN3CT Workshops 28 April 2017 Edward J. Plichta Chief Scientist for Power & Energy Command Power & Integration Directorate Aberdeen
BETTERY: An Italian startup for the design of novel redox flow batteries FRANCESCA DE GIORGIO - COFOUNDER
 BETTERY: An Italian startup for the design of novel redox flow batteries FRANCESCA DE GIORGIO - COFOUNDER SOLAR PV ELECTRIC MOBILITY WIND KEY TECHNOLOGY OPTIONS FOR THE ENERGY TRANSITION Accelerating the
BETTERY: An Italian startup for the design of novel redox flow batteries FRANCESCA DE GIORGIO - COFOUNDER SOLAR PV ELECTRIC MOBILITY WIND KEY TECHNOLOGY OPTIONS FOR THE ENERGY TRANSITION Accelerating the
Requirement, Design, and Challenges in Inorganic Solid State Batteries
 Requirement, Design, and Challenges in Inorganic Solid State Batteries Venkat Anandan Energy Storage Research Department 1 Ford s Electrified Vehicle Line-up HEV Hybrid Electric Vehicle C-Max Hybrid Fusion
Requirement, Design, and Challenges in Inorganic Solid State Batteries Venkat Anandan Energy Storage Research Department 1 Ford s Electrified Vehicle Line-up HEV Hybrid Electric Vehicle C-Max Hybrid Fusion
FRAUNHOFER INSTITUTE FOR CHEMICAL TECHNOLOGY ICT REDOX-FLOW BATTERY
 FRAUNHOFER INSTITUTE FOR CHEMICAL TECHNOLOGY ICT REDOX-FLOW BATTERY REDOX-FLOW BATTERY REDOX-FLOW BATTERY Redox-flow batteries are efficient and have a longer service life than conventional batteries.
FRAUNHOFER INSTITUTE FOR CHEMICAL TECHNOLOGY ICT REDOX-FLOW BATTERY REDOX-FLOW BATTERY REDOX-FLOW BATTERY Redox-flow batteries are efficient and have a longer service life than conventional batteries.
Li-ion Technology Overview NTSB Hearing Washington, D.C. July 12-13, 2006
 Li-ion Technology Overview NTSB Hearing Washington, D.C. July 12-13, 2006 Jason Howard, Ph.D. Distinguished Member of the Technical Staff, Motorola, Inc. Board of Directors, Portable Rechargeable Battery
Li-ion Technology Overview NTSB Hearing Washington, D.C. July 12-13, 2006 Jason Howard, Ph.D. Distinguished Member of the Technical Staff, Motorola, Inc. Board of Directors, Portable Rechargeable Battery
Battery technologies and their applications in sustainable developments. Dr. Denis Y.W. Yu Assistant Professor School of Energy and Environment
 Battery technologies and their applications in sustainable developments Dr. Denis Y.W. Yu Assistant Professor School of Energy and Environment May 29, 2014 Energy flow Energy Energy generation Energy storage
Battery technologies and their applications in sustainable developments Dr. Denis Y.W. Yu Assistant Professor School of Energy and Environment May 29, 2014 Energy flow Energy Energy generation Energy storage
Course of development of the lithium-ion battery (LIB), and recent technological trends
 Session 2A : Business Case Course of development of the lithium-ion (LIB), and recent technological trends Dr. Akira Yoshino Yoshino Laboratory Asahi Kasei Corp. E-mail: yoshino.ab@om.asahi-kasei.co.jp
Session 2A : Business Case Course of development of the lithium-ion (LIB), and recent technological trends Dr. Akira Yoshino Yoshino Laboratory Asahi Kasei Corp. E-mail: yoshino.ab@om.asahi-kasei.co.jp
Use of Aqueous Double Layer Ultracapacitor using Hybrid CDI-ED Technology for the use in Hybrid Battery Systmes
 Overview Use of Aqueous Double Layer Ultracapacitor using Hybrid CDI-ED Technology for the use in Hybrid Battery Systmes By Robert Atlas, Aqua EWP,LLC. September 2006 Aqua EWP. has for the last 10 years
Overview Use of Aqueous Double Layer Ultracapacitor using Hybrid CDI-ED Technology for the use in Hybrid Battery Systmes By Robert Atlas, Aqua EWP,LLC. September 2006 Aqua EWP. has for the last 10 years
Emerging Stationary Battery Technologies
 Emerging Stationary Battery Technologies Erik D. Spoerke, Ph.D. Sandia National Laboratories, Albuquerque, NM 2017 DLA Worldwide Energy Conference National Harbor, MD April 10-12, 2017 Sandia National
Emerging Stationary Battery Technologies Erik D. Spoerke, Ph.D. Sandia National Laboratories, Albuquerque, NM 2017 DLA Worldwide Energy Conference National Harbor, MD April 10-12, 2017 Sandia National
Keeping up with the increasing demands for electrochemical energy storage
 Keeping up with the increasing demands for electrochemical energy storage Jeff Sakamoto 2015 Top of the learning curve: optimize current technology 2020 Frontiers of Li-ion technology: new materials 2030
Keeping up with the increasing demands for electrochemical energy storage Jeff Sakamoto 2015 Top of the learning curve: optimize current technology 2020 Frontiers of Li-ion technology: new materials 2030
Implementation and development of standards for Lithium-ion energy storage technologies within the South African context
 Implementation and development of standards for Lithium-ion energy storage technologies within the South African context by Nico Rust, Nelson Mandela University uyilo EMTIP uyilo emobility Technology Innovation
Implementation and development of standards for Lithium-ion energy storage technologies within the South African context by Nico Rust, Nelson Mandela University uyilo EMTIP uyilo emobility Technology Innovation
Congratulations, Dorothy!
 Congratulations, Dorothy! Battery Overview Steve Garland Kyle Jamieson Outline Why is this important? Brief history of batteries Basic chemistry Battery types and characteristics Case study: ThinkPad battery
Congratulations, Dorothy! Battery Overview Steve Garland Kyle Jamieson Outline Why is this important? Brief history of batteries Basic chemistry Battery types and characteristics Case study: ThinkPad battery
CSIRO Energy Storage Projects: David Lamb Low Emission Transport Theme Leader
 CSIRO Energy Storage Projects: David Lamb Low Emission Transport Theme Leader Energy Storage for Transport Three projects Safe, High-Performance Lithium-Metal Batteries Supercapacitors Ultrabattery 10
CSIRO Energy Storage Projects: David Lamb Low Emission Transport Theme Leader Energy Storage for Transport Three projects Safe, High-Performance Lithium-Metal Batteries Supercapacitors Ultrabattery 10
UN/SCETDG/47/INF.13/Rev.1
 Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification and Labelling of Chemicals New proper shipping name for rechargeable lithium metal batteries
Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification and Labelling of Chemicals New proper shipping name for rechargeable lithium metal batteries
DOE OVT Energy Storage R&D Overview
 DOE OVT Energy Storage R&D Overview David Howell Hybrid and electric vehicles, energy storage technologies and control systems National and international R&D-projects, research institutions and funding
DOE OVT Energy Storage R&D Overview David Howell Hybrid and electric vehicles, energy storage technologies and control systems National and international R&D-projects, research institutions and funding
LARGE-SCALE THIN FILM BATTERY
 NCCAVS Annual Symposium February 23, 2017 LARGE-SCALE THIN FILM BATTERY Ernest Demaray (Demaray LLC) & Pavel Khokhlov (SpectraPower LLC) SpectraPower High Energy Density Li-metal cells The 6.6Ah battery
NCCAVS Annual Symposium February 23, 2017 LARGE-SCALE THIN FILM BATTERY Ernest Demaray (Demaray LLC) & Pavel Khokhlov (SpectraPower LLC) SpectraPower High Energy Density Li-metal cells The 6.6Ah battery
Materials Design and Diagnosis for Rechargeable Battery Energy Storage
 Materials Design and Diagnosis for Rechargeable Battery Energy Storage Shirley Meng Department of NanoEngineering University of California San Diego The Challenge of Power vs. Energy Power& 1& 1& W& 10
Materials Design and Diagnosis for Rechargeable Battery Energy Storage Shirley Meng Department of NanoEngineering University of California San Diego The Challenge of Power vs. Energy Power& 1& 1& W& 10
Aalborg Universitet. Published in: ECS Transactions. DOI (link to publication from Publisher): / ecst. Publication date: 2015
 Aalborg Universitet Study on Self-discharge Behavior of Lithium-Sulfur Batteries Knap, Vaclav; Stroe, Daniel-Ioan; Swierczynski, Maciej Jozef; Teodorescu, Remus; Schaltz, Erik Published in: ECS Transactions
Aalborg Universitet Study on Self-discharge Behavior of Lithium-Sulfur Batteries Knap, Vaclav; Stroe, Daniel-Ioan; Swierczynski, Maciej Jozef; Teodorescu, Remus; Schaltz, Erik Published in: ECS Transactions
Large Format Lithium Power Cells for Demanding Hybrid Applications
 Large Format Lithium Power Cells for Demanding Hybrid Applications Adam J. Hunt Manager of Government Programs 2011 Joint Service Power Expo Power to Sustain Warfighter Dominance Myrtle Beach, SC May 4,
Large Format Lithium Power Cells for Demanding Hybrid Applications Adam J. Hunt Manager of Government Programs 2011 Joint Service Power Expo Power to Sustain Warfighter Dominance Myrtle Beach, SC May 4,
MAT4BAT summer school Battery industry prospective in Europe and new technologies. C. Chanson
 MAT4BAT summer school Battery industry prospective in Europe and new technologies C. Chanson June 4, 2015 1 RECHARGE Membership throughout the Value Chain 2 RECHARGE Mission RECHARGE s mission is to promote
MAT4BAT summer school Battery industry prospective in Europe and new technologies C. Chanson June 4, 2015 1 RECHARGE Membership throughout the Value Chain 2 RECHARGE Mission RECHARGE s mission is to promote
New proper shipping name for rechargeable lithium metal batteries
 Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification and Labelling of Chemicals New proper shipping name for rechargeable lithium metal batteries
Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification and Labelling of Chemicals New proper shipping name for rechargeable lithium metal batteries
Batteries generally classifies into two main groups: primary and secondary battery types. Primary batteries are
 Battery types Batteries generally classifies into two main groups: primary and secondary battery types. Primary batteries are disposable batteries that cannot be recycled, and the secondary is the rechargeable
Battery types Batteries generally classifies into two main groups: primary and secondary battery types. Primary batteries are disposable batteries that cannot be recycled, and the secondary is the rechargeable
Cathode material for batteries the safe bridge to e-mobility
 Innovation Spotlight Life Power P2 Andrew Silver Cathode material for batteries the safe bridge to e-mobility Issue: Summer 2012 Lithium iron phosphate is at present the only inherently safe cathode material
Innovation Spotlight Life Power P2 Andrew Silver Cathode material for batteries the safe bridge to e-mobility Issue: Summer 2012 Lithium iron phosphate is at present the only inherently safe cathode material
The Discussion of this exercise covers the following points:
 Exercise 1 Battery Fundamentals EXERCISE OBJECTIVE When you have completed this exercise, you will be familiar with various types of lead-acid batteries and their features. DISCUSSION OUTLINE The Discussion
Exercise 1 Battery Fundamentals EXERCISE OBJECTIVE When you have completed this exercise, you will be familiar with various types of lead-acid batteries and their features. DISCUSSION OUTLINE The Discussion
Li-Ion battery Model. Octavio Salazar. Octavio Salazar
 Li-Ion battery Model 1 Energy Storage- Lithium Ion Batteries C-PCS: Control and Power Conditioning System Energy Storage- Lithium Ion Batteries Nature [0028-0836] Tarascon (2001) volume: 414 issue: 6861
Li-Ion battery Model 1 Energy Storage- Lithium Ion Batteries C-PCS: Control and Power Conditioning System Energy Storage- Lithium Ion Batteries Nature [0028-0836] Tarascon (2001) volume: 414 issue: 6861
Argonne Mobility Research Impending Electrification. Don Hillebrand Argonne National Laboratory
 Argonne Mobility Research Impending Electrification Don Hillebrand Argonne National Laboratory 2018 Argonne: DOE s Largest Transportation Research Program Located 25 miles from the Chicago Loop, Argonne
Argonne Mobility Research Impending Electrification Don Hillebrand Argonne National Laboratory 2018 Argonne: DOE s Largest Transportation Research Program Located 25 miles from the Chicago Loop, Argonne
Review of status of the main chemistries for the EV market
 Review of status of the main chemistries for the EV market EMIRI Energy Materials Industrial Research Initiative Dr. Marcel Meeus Consultant Sustesco www.emiri.eu 1 Agenda 1. Review of status of current
Review of status of the main chemistries for the EV market EMIRI Energy Materials Industrial Research Initiative Dr. Marcel Meeus Consultant Sustesco www.emiri.eu 1 Agenda 1. Review of status of current
Development of High Power Li-ion Cell "LIM25H" for Industrial Applications
 Technical Report 報文 Development of High Power Li-ion Cell "" for Industrial Applications Yasushi Uebo * Keiji Shimomura * Katsushi Nishie * Katsuya Nanamoto * Takehito Matsubara ** Haruo Seike ** Minoru
Technical Report 報文 Development of High Power Li-ion Cell "" for Industrial Applications Yasushi Uebo * Keiji Shimomura * Katsushi Nishie * Katsuya Nanamoto * Takehito Matsubara ** Haruo Seike ** Minoru
BATTERIES SODIUM, POTASSIUM, SILICON
 BATTERIES SODIUM, POTASSIUM, SILICON Introduction Energy is a key for scientists, business, and policy makers. Energy storage is a need. This need is due to the non-continuous working hours of rising energy
BATTERIES SODIUM, POTASSIUM, SILICON Introduction Energy is a key for scientists, business, and policy makers. Energy storage is a need. This need is due to the non-continuous working hours of rising energy
Course Syllabus and Information
 Energy Storage Systems for Electric-based Transportations Course Syllabus and Information College of Engineering Department of Electrical and Computer Engineering Course No. ECE-5995 Selected topics Winter
Energy Storage Systems for Electric-based Transportations Course Syllabus and Information College of Engineering Department of Electrical and Computer Engineering Course No. ECE-5995 Selected topics Winter
Clean energy systems need clean batteries
 Energy Storage. Clean and Simple. Clean energy systems need clean batteries Introducing the first clean and sustainable battery Our batteries are different. Hassle-free Peace of Mind How We Stack Up AHI
Energy Storage. Clean and Simple. Clean energy systems need clean batteries Introducing the first clean and sustainable battery Our batteries are different. Hassle-free Peace of Mind How We Stack Up AHI
Aqueous Rechargeable Lithium Batteries (ARLBs) of High Energy Density. Prof. Dr. Yuping Wu
 Aqueous Rechargeable Lithium Batteries (ARLBs) of High Energy Density Prof. Dr. Yuping Wu New Energy and Materials Laboratory (NEML), Department of Chemistry, Fudan University, Shanghai 200433 Tel/Fax:
Aqueous Rechargeable Lithium Batteries (ARLBs) of High Energy Density Prof. Dr. Yuping Wu New Energy and Materials Laboratory (NEML), Department of Chemistry, Fudan University, Shanghai 200433 Tel/Fax:
From materials to vehicle what, why, and how? From vehicle to materials
 From materials to vehicle what, why, and how? From vehicle to materials Helena Berg Outline 1. Electric vehicles and requirements 2. Battery packs for vehicles 3. Cell selection 4. Material requirements
From materials to vehicle what, why, and how? From vehicle to materials Helena Berg Outline 1. Electric vehicles and requirements 2. Battery packs for vehicles 3. Cell selection 4. Material requirements
Introduction. Today, we can convert energy from many different forms into usable electricity.
 Introduction Today, we can convert energy from many different forms into usable electricity. But how did we get here? In ancient times, the generation of electricity was purely accidental. 1. Drag feet
Introduction Today, we can convert energy from many different forms into usable electricity. But how did we get here? In ancient times, the generation of electricity was purely accidental. 1. Drag feet
Nickel-Zinc Large Format Batteries for Military Ground Vehicles
 2010 NDIA GROUND VEHICLE SYSTEMS ENGINEERING AND TECHNOLOGY SYMPOSIUM POWER AND ENERGY (P&E) MINI-SYMPOSIUM AUGUST 17-19 DEARBORN, MICHIGAN Todd Tatar, Jeff Philips, Salil Soman, and Richard Brody PowerGenix
2010 NDIA GROUND VEHICLE SYSTEMS ENGINEERING AND TECHNOLOGY SYMPOSIUM POWER AND ENERGY (P&E) MINI-SYMPOSIUM AUGUST 17-19 DEARBORN, MICHIGAN Todd Tatar, Jeff Philips, Salil Soman, and Richard Brody PowerGenix
I. Equivalent Circuit Models Lecture 3: Electrochemical Energy Storage
 I. Equivalent Circuit Models Lecture 3: Electrochemical Energy Storage MIT Student In this lecture, we will learn some examples of electrochemical energy storage. A general idea of electrochemical energy
I. Equivalent Circuit Models Lecture 3: Electrochemical Energy Storage MIT Student In this lecture, we will learn some examples of electrochemical energy storage. A general idea of electrochemical energy
Ionic Additives for Electrochemical Devices Using Intercalation Electrodes
 U.S. Army Research, Development and Engineering Command Ionic Additives for Electrochemical Devices Using Intercalation Electrodes Inventor: Dr. Kang Xu ARL 09-18 February 16, 2011 Technology Overview
U.S. Army Research, Development and Engineering Command Ionic Additives for Electrochemical Devices Using Intercalation Electrodes Inventor: Dr. Kang Xu ARL 09-18 February 16, 2011 Technology Overview
Supercaps Fields of Application and Limits
 Supercaps Fields of Application and Limits Dietmar Rahner TU Dresden Institut für Physikalische Chemie und Elektrochemie D-01062 Dresden Steffen Rahner Battery-Lab Rahner GmbH Dresden D-01217 Dresden www.battery-lab.de
Supercaps Fields of Application and Limits Dietmar Rahner TU Dresden Institut für Physikalische Chemie und Elektrochemie D-01062 Dresden Steffen Rahner Battery-Lab Rahner GmbH Dresden D-01217 Dresden www.battery-lab.de
E lectrical energy storage has become an
 Addressing the Grand Challenges in Energy Storage Jun Liu * E lectrical energy storage has become an important topic of discussion and debate for both automobiles (transportation) and electrical grid (stationary)
Addressing the Grand Challenges in Energy Storage Jun Liu * E lectrical energy storage has become an important topic of discussion and debate for both automobiles (transportation) and electrical grid (stationary)
Studies on Capacity Fade of Spinel-Based Li-Ion Batteries
 A54 0013-4651/2001/149 1 /A54/7/$7.00 The Electrochemical Society, Inc. Studies on Capacity Fade of Spinel-Based Li-Ion Batteries Ramadass Premanand, Anand Durairajan,* Bala Haran,** Ralph White,*** and
A54 0013-4651/2001/149 1 /A54/7/$7.00 The Electrochemical Society, Inc. Studies on Capacity Fade of Spinel-Based Li-Ion Batteries Ramadass Premanand, Anand Durairajan,* Bala Haran,** Ralph White,*** and
UN/SCETDG/52/INF.11. Sodium-Ion Batteries. Introduction
 Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification and Labelling of Chemicals UN/SCETDG/52/INF.11 Sub-Committee of Experts on the Transport
Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification and Labelling of Chemicals UN/SCETDG/52/INF.11 Sub-Committee of Experts on the Transport
Sundar Mayavan Lead Acid Battery Group CSIR- Central Electrochemical Research Institute Karaikudi, INDIA. 22/9/ th Asian Battery Conference 1
 Influence of Carbon Nanotubes, Glycine, Boron-Carbon- Nitride and Molybdenum disulfide as Negative-Plate Additives on the Performance of Lead Acid Batteries Sundar Mayavan Lead Acid Battery Group CSIR-
Influence of Carbon Nanotubes, Glycine, Boron-Carbon- Nitride and Molybdenum disulfide as Negative-Plate Additives on the Performance of Lead Acid Batteries Sundar Mayavan Lead Acid Battery Group CSIR-
Development of a rechargeable zinc-air battery
 1.1149/1.357924 The Electrochemical Society Development of a rechargeable zinc-air battery Gwenaëlle Toussaint* a, Philippe Stevens a, Laurent Akrour a, Robert Rouget b and Fabrice Fourgeot b a EDF R&D,
1.1149/1.357924 The Electrochemical Society Development of a rechargeable zinc-air battery Gwenaëlle Toussaint* a, Philippe Stevens a, Laurent Akrour a, Robert Rouget b and Fabrice Fourgeot b a EDF R&D,
BATTERIES & SUPERCAPS POST MORTEM ANALYSIS PLATFORM EXTERNAL SERVICES
 BATTERIES & SUPERCAPS POST MORTEM ANALYSIS PLATFORM EXTERNAL SERVICES CONTEXT Over the last years a remarkable evolution has taken place by the introduction of new batteries & supercapacitors technologies
BATTERIES & SUPERCAPS POST MORTEM ANALYSIS PLATFORM EXTERNAL SERVICES CONTEXT Over the last years a remarkable evolution has taken place by the introduction of new batteries & supercapacitors technologies
Vehicle Battery R&D Progress and Future Plans
 Vehicle Battery R&D Progress and Future Plans Tien Q. Duong Office of Vehicle Technologies U.S. Department of Energy KSAE and IEA IA-HEV International Symposium on Electric Mobility and IA-HEV Task 1 Information
Vehicle Battery R&D Progress and Future Plans Tien Q. Duong Office of Vehicle Technologies U.S. Department of Energy KSAE and IEA IA-HEV International Symposium on Electric Mobility and IA-HEV Task 1 Information
FACETS OF GRAPHITE. June 2017
 FACETS OF GRAPHITE June 2017 1. INTRODUCTION What is Graphite? Why is Graphite Important? Current Demand & Prices for Selected High Purity Graphite Applications Contents 2. SELECTED APPLICATIONS Lithium
FACETS OF GRAPHITE June 2017 1. INTRODUCTION What is Graphite? Why is Graphite Important? Current Demand & Prices for Selected High Purity Graphite Applications Contents 2. SELECTED APPLICATIONS Lithium
A Structure of Cylindrical Lithium-ion Batteries
 Introduction A Structure of Cylindrical Lithium-ion Batteries A lithium-ion battery is an energy storage device providing electrical energy by using chemical reactions. A few types of lithium-ion battery
Introduction A Structure of Cylindrical Lithium-ion Batteries A lithium-ion battery is an energy storage device providing electrical energy by using chemical reactions. A few types of lithium-ion battery
Batteries for electric commercial vehicles and mobile machinery
 Batteries for electric commercial vehicles and mobile machinery Tekes EVE annual seminar, Dipoli 6.11.2012 Dr. Mikko Pihlatie VTT Technical Research Centre of Finland 2 Outline 1. Battery technology for
Batteries for electric commercial vehicles and mobile machinery Tekes EVE annual seminar, Dipoli 6.11.2012 Dr. Mikko Pihlatie VTT Technical Research Centre of Finland 2 Outline 1. Battery technology for
Seoul, Korea. 6 June 2018
 Seoul, Korea 6 June 2018 Innovation roadmap in clean mobility materials SPEAKER Denis Goffaux Chief Technology Officer Executive Vice-President Energy & Surface Technologies 2 Agenda Well to wheel efficiency
Seoul, Korea 6 June 2018 Innovation roadmap in clean mobility materials SPEAKER Denis Goffaux Chief Technology Officer Executive Vice-President Energy & Surface Technologies 2 Agenda Well to wheel efficiency
Guidelines for Battery Electric Vehicles in the Underground
 Guidelines for Battery Electric Vehicles in the Underground Energy Storage Systems Rich Zajkowski Energy Storage Safety & Compliance Eng. GE Transportation Agenda Terminology Let s Design a Battery System
Guidelines for Battery Electric Vehicles in the Underground Energy Storage Systems Rich Zajkowski Energy Storage Safety & Compliance Eng. GE Transportation Agenda Terminology Let s Design a Battery System
Leveraging developments in xev Lithium batteries for stationary applications
 Leveraging developments in xev Lithium batteries for stationary applications International Colloquium on Energy Storage Brussels, Nov 8 th, 2017 Daniel Gloesener Global technical leader- Battery Technologies,
Leveraging developments in xev Lithium batteries for stationary applications International Colloquium on Energy Storage Brussels, Nov 8 th, 2017 Daniel Gloesener Global technical leader- Battery Technologies,
July 5, 2017 MEMORANDUM. Power Committee. Massoud Jourabchi. SUBJECT: Report on Life-cycle of Batteries BACKGROUND: Presenters: Massoud Jourabchi
 Henry Lorenzen Chair Oregon Bill Bradbury Oregon Guy Norman Washington Tom Karier Washington W. Bill Booth Vice Chair Idaho James Yost Idaho Jennifer Anders Montana Tim Baker Montana July 5, 2017 MEMORANDUM
Henry Lorenzen Chair Oregon Bill Bradbury Oregon Guy Norman Washington Tom Karier Washington W. Bill Booth Vice Chair Idaho James Yost Idaho Jennifer Anders Montana Tim Baker Montana July 5, 2017 MEMORANDUM
PERFORMANCE CHARACTERIZATION OF NICD BATTERY BY ARBIN BT2000 ANALYZER IN BATAN
 MATERIALS SCIENCE and TECHNOLOGY Edited by Evvy Kartini et.al. PERFORMANCE CHARACTERIZATION OF NICD BATTERY BY ARBIN BT2000 ANALYZER IN BATAN H. Jodi, E. Kartini, T. Nugraha Center for Technology of Nuclear
MATERIALS SCIENCE and TECHNOLOGY Edited by Evvy Kartini et.al. PERFORMANCE CHARACTERIZATION OF NICD BATTERY BY ARBIN BT2000 ANALYZER IN BATAN H. Jodi, E. Kartini, T. Nugraha Center for Technology of Nuclear
Supercapacitors. 1. Principle of operation and physical models 2. Materials used in supercapacitors 3. Applications
 Supercapacitors 1. Principle of operation and physical models 2. Materials used in supercapacitors 3. Applications Capacitors Electrical capacitance C = Q U U = D 0 E( x) dx Flat capacitor C = Sεε 0 D
Supercapacitors 1. Principle of operation and physical models 2. Materials used in supercapacitors 3. Applications Capacitors Electrical capacitance C = Q U U = D 0 E( x) dx Flat capacitor C = Sεε 0 D
Innovative Uses of Nickel. Joint Study Groups Seminar New & Innovative Applications for Metals. 28 April 2010 Lisbon, Portugal
 Innovative Uses of Nickel Joint Study Groups Seminar New & Innovative Applications for Metals 28 April 2010 Lisbon, Portugal Innovative Uses of Nickel Innovative Projects Incorporate Nickel In transportation
Innovative Uses of Nickel Joint Study Groups Seminar New & Innovative Applications for Metals 28 April 2010 Lisbon, Portugal Innovative Uses of Nickel Innovative Projects Incorporate Nickel In transportation
EE Chapter 2 Aircraft Storage Batteries
 EE 2145230 Chapter 2 Aircraft Storage Batteries Two types of batteries used on nearly all aircraft are nickel cadmium and lead acid batteries. All batteries produce dc voltage. 2.1 Dry Cells and Batteries
EE 2145230 Chapter 2 Aircraft Storage Batteries Two types of batteries used on nearly all aircraft are nickel cadmium and lead acid batteries. All batteries produce dc voltage. 2.1 Dry Cells and Batteries
Introduction to Solar Electric Battery Systems. J-Tech Solar Training
 Introduction to Solar Electric Battery Systems J-Tech Solar Training Instructor Biography Jim Parish Jim has been involved in the Solar Industry for over 15 years. He designed and installed the first Photovoltaic
Introduction to Solar Electric Battery Systems J-Tech Solar Training Instructor Biography Jim Parish Jim has been involved in the Solar Industry for over 15 years. He designed and installed the first Photovoltaic
Study on the Performance of Lithium-Ion Batteries at Different Temperatures Shanshan Guo1,a*,Yun Liu1,b and Lin Li2,c 1
 7th International Conference on Mechatronics, Computer and Education Informationization (MCEI 217) Study on the Performance of Lithium-Ion Batteries at Different Temperatures Shanshan Guo1,a*,Yun Liu1,b
7th International Conference on Mechatronics, Computer and Education Informationization (MCEI 217) Study on the Performance of Lithium-Ion Batteries at Different Temperatures Shanshan Guo1,a*,Yun Liu1,b
The BEEST: An Overview of ARPA-E s Program in Ultra-High Energy Batteries for Electrified Vehicles
 The BEEST: An Overview of ARPA-E s Program in Ultra-High Energy Batteries for Electrified Vehicles David Danielson, PhD Program Director, ARPA-E NDIA Workshop to Catalyze Adoption of Next-Generation Energy
The BEEST: An Overview of ARPA-E s Program in Ultra-High Energy Batteries for Electrified Vehicles David Danielson, PhD Program Director, ARPA-E NDIA Workshop to Catalyze Adoption of Next-Generation Energy
16 1 Vol. 16 No ELECTROCHEMISTRY Feb. 2010
 16 1 Vol 16 No 1 2010 2 ELECTROCHEMISTRY Feb 2010 1006-3471 2010 01-0006-05 Ⅰ * 430072 O646 21 TM911 A 1 3-4 1 120 SEI 1 2 3 2009-11-10 2009-12-14 Tel 86-27 68754526 E-mail xpai@ whu edu cn 973 No 2009CB220103
16 1 Vol 16 No 1 2010 2 ELECTROCHEMISTRY Feb 2010 1006-3471 2010 01-0006-05 Ⅰ * 430072 O646 21 TM911 A 1 3-4 1 120 SEI 1 2 3 2009-11-10 2009-12-14 Tel 86-27 68754526 E-mail xpai@ whu edu cn 973 No 2009CB220103
Safeguarding lithium-ion battery cell separators
 Safeguarding lithium-ion battery cell separators Executive Summary Technical advances in the design and construction of lithium-ion battery cells have played an essential role in the widespread deployment
Safeguarding lithium-ion battery cell separators Executive Summary Technical advances in the design and construction of lithium-ion battery cells have played an essential role in the widespread deployment
Vanadium-Bromine Redox Flow Battery
 Vanadium-Bromine Redox Flow Battery Flow Batterie Kolloquium in Karlsruhe am 27. September 2017 H. Frank Gibbard, Ph.D. CEO WattJoule Corporation Devens, Massachusetts USA Stationary Energy Storage Why
Vanadium-Bromine Redox Flow Battery Flow Batterie Kolloquium in Karlsruhe am 27. September 2017 H. Frank Gibbard, Ph.D. CEO WattJoule Corporation Devens, Massachusetts USA Stationary Energy Storage Why
Batteries: Stored Energy Discussion Questions:
 Batteries: Stored Energy Discussion Questions: 1) How is energy stored in a battery? 2) How many different types of batteries are there? 3) What kinds of tools and machinery can run on batteries? 4) Can
Batteries: Stored Energy Discussion Questions: 1) How is energy stored in a battery? 2) How many different types of batteries are there? 3) What kinds of tools and machinery can run on batteries? 4) Can
Unit 13 Batteries and Other Electrical Sources
 Batteries and Other Electrical Sources Objectives: Discuss the differences between primary and secondary cells. List voltages for different types of cells. Discuss different types of primary cells. Construct
Batteries and Other Electrical Sources Objectives: Discuss the differences between primary and secondary cells. List voltages for different types of cells. Discuss different types of primary cells. Construct
APPLIED ELECTROCHEMISTRY Technion s Chemical Power Sources Research
 ה ט כ נ י ו ן מ כ ו ן ט כ נ ו ל ו ג י ל י ש ר א ל TECHNION - ISRAEL INSTITUTE OF TECHNOLOGY הפקולטה למדע והנדסה של חומרים DEPARTMENT OF MATERIALS SCIENCE & ENGINEERING - APPLIED ELECTROCHEMISTRY Technion
ה ט כ נ י ו ן מ כ ו ן ט כ נ ו ל ו ג י ל י ש ר א ל TECHNION - ISRAEL INSTITUTE OF TECHNOLOGY הפקולטה למדע והנדסה של חומרים DEPARTMENT OF MATERIALS SCIENCE & ENGINEERING - APPLIED ELECTROCHEMISTRY Technion
Prototype Micro Fuel Cell for FOMA Terminals
 Prototype Micro Fuel Cell for FOMA Terminals Kazuhiko Takeno, Takayuki Kanai and Remi Shirota As FOMA terminals become increasingly sophisticated, they consume more power. We have investigated and manufactured
Prototype Micro Fuel Cell for FOMA Terminals Kazuhiko Takeno, Takayuki Kanai and Remi Shirota As FOMA terminals become increasingly sophisticated, they consume more power. We have investigated and manufactured
Introduction. Analysis
 10/21/2017 Memorandum To: Mike Kozicki, CTO, Second Solar, Inc. From: Athena Combs-Hurtado and Marc Hensel Re: Using Lithium Iron Phosphate Batteries for Utility Scale Storage Applications Introduction
10/21/2017 Memorandum To: Mike Kozicki, CTO, Second Solar, Inc. From: Athena Combs-Hurtado and Marc Hensel Re: Using Lithium Iron Phosphate Batteries for Utility Scale Storage Applications Introduction
Lithium Coin Handbook and Application Manual
 : Lithium coin cells were originally developed in the 1970 s as a 3 volt miniature power source for low drain and battery backup applications. Their high energy density and long shelf life made them well
: Lithium coin cells were originally developed in the 1970 s as a 3 volt miniature power source for low drain and battery backup applications. Their high energy density and long shelf life made them well
Energy Storage. Chm446/1304 April 2, 2014 Hand your assignments in at the front.
 Energy Storage Chm446/1304 April 2, 2014 Hand your assignments in at the front http://www.youtube.com/watch?v=dtqsiplgxa&feature=youtu.be World Energy Needs Projected to Increase 53% From 2008 to 2035
Energy Storage Chm446/1304 April 2, 2014 Hand your assignments in at the front http://www.youtube.com/watch?v=dtqsiplgxa&feature=youtu.be World Energy Needs Projected to Increase 53% From 2008 to 2035
Survey of Commercial Small Lithium Polymer Batteries
 Naval Research Laboratory Washington, DC 20375-5320 NRL/MR/6110--07-9073 Survey of Commercial Small Lithium Polymer Batteries Arnold M. Stux Karen Swider-Lyons Chemical Dynamics and Diagnostics Branch
Naval Research Laboratory Washington, DC 20375-5320 NRL/MR/6110--07-9073 Survey of Commercial Small Lithium Polymer Batteries Arnold M. Stux Karen Swider-Lyons Chemical Dynamics and Diagnostics Branch
Understanding Lithium-Ion Technology Jim McDowall (updated from Battcon 2008)
 Understanding Lithium-Ion Technology Jim McDowall (updated from Battcon 2008) PE/SB Winter Meeting 2015, New Orleans Background History Started with primary batteries with metallic lithium negatives True
Understanding Lithium-Ion Technology Jim McDowall (updated from Battcon 2008) PE/SB Winter Meeting 2015, New Orleans Background History Started with primary batteries with metallic lithium negatives True
THINERGY MEC220. Solid-State, Flexible, Rechargeable Thin-Film Micro-Energy Cell
 THINERGY MEC220 Solid-State, Flexible, Rechargeable Thin-Film Micro-Energy Cell DS1013 v1.1 Preliminary Product Data Sheet Features Thin Form Factor 170 µm Thick Capacity options up to 400 µah All Solid-State
THINERGY MEC220 Solid-State, Flexible, Rechargeable Thin-Film Micro-Energy Cell DS1013 v1.1 Preliminary Product Data Sheet Features Thin Form Factor 170 µm Thick Capacity options up to 400 µah All Solid-State
UN Transportation Tests and UL Lithium Battery Program
 UN Transportation Tests and UL Lithium Battery Program Underwriters Laboratories Inc. - General Experience and Status Update November 11, 2008 Copyright 1995-2007 Underwriters Laboratories Inc. All rights
UN Transportation Tests and UL Lithium Battery Program Underwriters Laboratories Inc. - General Experience and Status Update November 11, 2008 Copyright 1995-2007 Underwriters Laboratories Inc. All rights
10 MINUTE LTO ULTRAFAST CHARGE PUBLIC TRANSIT EV BUS FLEET OPERATIONAL DATA - ANALYSIS OF 240,000 KM, 6 BUS FLEET SHOWS VIABLE SOLUTION"
 World Electric Vehicle Journal Vol. 5 - ISSN 2032-6653 - 2012 WEVA Page 0261 EVS26 Los Angeles, California, May 6-9, 2012 10 MINUTE LTO ULTRAFAST CHARGE PUBLIC TRANSIT EV BUS FLEET OPERATIONAL DATA - ANALYSIS
World Electric Vehicle Journal Vol. 5 - ISSN 2032-6653 - 2012 WEVA Page 0261 EVS26 Los Angeles, California, May 6-9, 2012 10 MINUTE LTO ULTRAFAST CHARGE PUBLIC TRANSIT EV BUS FLEET OPERATIONAL DATA - ANALYSIS
AARHUS UNIVERSITET FLOW BATTERIER PÅ VEJ IND I KOMMERCIEL DANSK SERIEPRODUKTION
 FLOW BATTERIER PÅ VEJ IND I KOMMERCIEL DANSK SERIEPRODUKTION Background Associate Professor Department of Engineering -Research in batteries and solar energy conversion Co-founder of VisBlue commercialisation
FLOW BATTERIER PÅ VEJ IND I KOMMERCIEL DANSK SERIEPRODUKTION Background Associate Professor Department of Engineering -Research in batteries and solar energy conversion Co-founder of VisBlue commercialisation
Available online at ScienceDirect. 21st CIRP Conference on Life Cycle Engineering
 Available online at www.sciencedirect.com ScienceDirect Procedia CIRP 15 ( 2014 ) 218 222 21st CIRP Conference on Life Cycle Engineering A method for pre-determining the optimal remanufacturing point of
Available online at www.sciencedirect.com ScienceDirect Procedia CIRP 15 ( 2014 ) 218 222 21st CIRP Conference on Life Cycle Engineering A method for pre-determining the optimal remanufacturing point of
Field Test of Green Base Station Designed for Environmental Friendliness and Reliability during Disasters. Research Laboratories
 Green Base Station Solar Power Generation Remote Control Field Test of Green Base Station Designed for Environmental Friendliness and Reliability during Disasters NTT DOCOMO Technical Journal 1. Introduction
Green Base Station Solar Power Generation Remote Control Field Test of Green Base Station Designed for Environmental Friendliness and Reliability during Disasters NTT DOCOMO Technical Journal 1. Introduction
12-Batteries and Inverters. ECEGR 452 Renewable Energy Systems
 12-Batteries and Inverters ECEGR 452 Renewable Energy Systems Overview Batteries Lead-Acid Batteries Battery Specifications Battery Charge Controllers Inverters Dr. Louie 2 Batteries Incorporation of a
12-Batteries and Inverters ECEGR 452 Renewable Energy Systems Overview Batteries Lead-Acid Batteries Battery Specifications Battery Charge Controllers Inverters Dr. Louie 2 Batteries Incorporation of a
FUEL CELLS AND BATTERIES LECTURE NO. 9
 SECONDARY BATTERIES Secondary or rechargeable batteries are widely used in many applications. The most familiar are starting, lighting, and ignition (SLI) automotive applications; industrial truck materials
SECONDARY BATTERIES Secondary or rechargeable batteries are widely used in many applications. The most familiar are starting, lighting, and ignition (SLI) automotive applications; industrial truck materials
Energy Storage. Electrochemical Cells & Batteries
 Energy Storage These notes cover the different methods that can be employed to store energy in various forms. These notes cover the storage of Electrical Energy, Kinetic Energy, and Pneumatic Energy. There
Energy Storage These notes cover the different methods that can be employed to store energy in various forms. These notes cover the storage of Electrical Energy, Kinetic Energy, and Pneumatic Energy. There
SAE BATTERY RECYCLING COMMITTEE: BATTERY RECYCLING APPROACHES FOR THE 21 ST CENTURY. Colin Pelletier, Timothy Ellis RSR Technologies Dallas, TX
 SAE BATTERY RECYCLING COMMITTEE: BATTERY RECYCLING APPROACHES FOR THE 21 ST CENTURY Colin Pelletier, Timothy Ellis RSR Technologies Dallas, TX Battery Recycling Committee: What is the Mission Mission:
SAE BATTERY RECYCLING COMMITTEE: BATTERY RECYCLING APPROACHES FOR THE 21 ST CENTURY Colin Pelletier, Timothy Ellis RSR Technologies Dallas, TX Battery Recycling Committee: What is the Mission Mission:
Dye sensitized solar cells - a successful research
 Dye sensitized solar cells - a successful research Adélio Mendes Porto & FEUP, February 23, 2016 Chemical Engineering Department Portugal & Porto Porto Lisbon 2 Faculty of Engineering Chemical Engineering
Dye sensitized solar cells - a successful research Adélio Mendes Porto & FEUP, February 23, 2016 Chemical Engineering Department Portugal & Porto Porto Lisbon 2 Faculty of Engineering Chemical Engineering
Zinc-Air Batteries for UAVs and MAVs
 Zinc-Air Batteries for UAVs and MAVs Dr. Neal Naimer, Vice President R&D (speaker) Binyamin Koretz, Vice President Business Development Ronald Putt, Director of Technology Electric Fuel Corporation Auburn,
Zinc-Air Batteries for UAVs and MAVs Dr. Neal Naimer, Vice President R&D (speaker) Binyamin Koretz, Vice President Business Development Ronald Putt, Director of Technology Electric Fuel Corporation Auburn,
THE IMPACT OF BATTERY OPERATING TEMPERATURE AND STATE OF CHARGE ON THE LITHIUM-ION BATTERY INTERNAL RESISTANCE
 Jurnal Mekanikal June 2017, Vol 40, 01-08 THE IMPACT OF BATTERY OPERATING TEMPERATURE AND STATE OF CHARGE ON THE LITHIUM-ION BATTERY INTERNAL RESISTANCE Amirul Haniff Mahmud, Zul Hilmi Che Daud, Zainab
Jurnal Mekanikal June 2017, Vol 40, 01-08 THE IMPACT OF BATTERY OPERATING TEMPERATURE AND STATE OF CHARGE ON THE LITHIUM-ION BATTERY INTERNAL RESISTANCE Amirul Haniff Mahmud, Zul Hilmi Che Daud, Zainab
Lithium battery charging
 Lithium battery charging How to charge to extend battery life? Why Lithium? Compared with the traditional battery, lithium ion battery charge faster, last longer, and have a higher power density for more
Lithium battery charging How to charge to extend battery life? Why Lithium? Compared with the traditional battery, lithium ion battery charge faster, last longer, and have a higher power density for more
Battery materials investments. Marc Grynberg, CEO Kurt Vandeputte, Business Line Manager 31 March 2010
 Battery materials investments Marc Grynberg, CEO Kurt Vandeputte, Business Line Manager 31 March 2010 1 Investment summary Umicore to invest in new production and development capabilities in Japan, South
Battery materials investments Marc Grynberg, CEO Kurt Vandeputte, Business Line Manager 31 March 2010 1 Investment summary Umicore to invest in new production and development capabilities in Japan, South
The Challenges of Electric Energy Storage. Nigel Taylor, Nick Green, Chris Lyness, Steve Nicholls
 The Challenges of Electric Energy Storage Nigel Taylor, Nick Green, Chris Lyness, Steve Nicholls Technology Walk Customer familiarity with recharging IC HEV PHEV EV Kinetic energy recovery Plug-in Battery
The Challenges of Electric Energy Storage Nigel Taylor, Nick Green, Chris Lyness, Steve Nicholls Technology Walk Customer familiarity with recharging IC HEV PHEV EV Kinetic energy recovery Plug-in Battery
High Energy Rechargeable Li-S Battery Development at Sion Power and BASF
 High Energy Rechargeable Li-S Battery Development at Sion Power and BASF Y. Mikhaylik*, C. Scordilis-Kelley*, M. Safont*, M. Laramie*, R. Schmidt**, H. Schneider**, K. Leitner** *Sion Power Corporation,
High Energy Rechargeable Li-S Battery Development at Sion Power and BASF Y. Mikhaylik*, C. Scordilis-Kelley*, M. Safont*, M. Laramie*, R. Schmidt**, H. Schneider**, K. Leitner** *Sion Power Corporation,
Introducing the nanoflowcell
 Introducing the nanoflowcell Vaduz, 4 March 2014 Thanks to its nanoflowcell, a revolutionary further development of flow cell technology, will make it possible for the first time in history to power an
Introducing the nanoflowcell Vaduz, 4 March 2014 Thanks to its nanoflowcell, a revolutionary further development of flow cell technology, will make it possible for the first time in history to power an
Altairnano Grid Stability and Transportation Products
 Altairnano Grid Stability and Transportation Products Joe Heinzmann Senior Director Energy Storage Solutions 1 Altairnano Overview Altairnano is an emerging growth company which is developing and commercializing
Altairnano Grid Stability and Transportation Products Joe Heinzmann Senior Director Energy Storage Solutions 1 Altairnano Overview Altairnano is an emerging growth company which is developing and commercializing
Panasonic Develops Industry's First *1 Nickel-Cadmium Battery Operable at Minus 40 C
 FOR IMMEDIATE RELEASE Media Contacts: Tokyo Public Relations Office Panasonic Corporation Tel: +81-(0)3-3574-5664 Fax: +81-(0)3-3574-5699 Panasonic News Bureau Tel: +81-(0)3-3542-6205 Fax: +81-(0)3-3542-9018
FOR IMMEDIATE RELEASE Media Contacts: Tokyo Public Relations Office Panasonic Corporation Tel: +81-(0)3-3574-5664 Fax: +81-(0)3-3574-5699 Panasonic News Bureau Tel: +81-(0)3-3542-6205 Fax: +81-(0)3-3542-9018
