Production of a biodiesel-like biofuel without glycerol generation, by using Novozym 435, an immobilized Candida antarctica lipase
|
|
|
- Kathlyn O’Connor’
- 5 years ago
- Views:
Transcription
1 Luna et al. Bioresources and Bioprocessing 2014, 1:11 RESEARCH Open Access Production of a biodiesel-like biofuel without glycerol generation, by using Novozym 435, an immobilized Candida antarctica lipase Carlos Luna 1, Cristóbal Verdugo 2, Enrique D Sancho 3, Diego Luna 1,4*, Juan Calero 1, Alejandro Posadillo 4, Felipa M Bautista 1 and Antonio A Romero 1 Abstract Background: Novozym 435, a commercial lipase from Candida antarctica, recombinant, expressed in Aspergillus niger, immobilized on macroporous acrylic resin, has been already described in the obtention of biodiesel. It is here evaluated in the production of a new biofuel that integrates the glycerol as monoglyceride (MG) together with two fatty acid ethyl esters (FAEE) molecules by the application of 1,3-selective lipases in the ethanolysis reaction of sunflower oil. Results: Response surface methodology (RSM) is employed to estimate the effects of main reaction. Optimum conditions for the viscosity, selectivity, and conversion were determined using a multifactorial design of experiments with three factors run by the software Stat Graphics version XV.I. The selected experimental parameters were reaction temperature, oil/ethanol ratio and alkaline environment. On the basis of RSM analysis, the optimum conditions for synthesis were 1/6 oil/etoh molar ratio, 30 C, and 12.5 μl of NaOH 10 N aqueous solutions, higher stirring than 300 rpm, for 2 h and 0.5 g of biocatalyst. Conclusions: These obtained results have proven a very good efficiency of the biocatalyst in the studied selective process. Furthermore, it was allowed sixteen times the successive reuse of the biocatalyst with good performance. Keywords: Biodiesel; Enzyme biocatalysis; Candida antarctica lipase B (CALB); N435; Response surface methodology; Transesterification Background Currently, fossil fuel is globally the main primary source of energy. However, as its availability is becoming increasingly limited, it is accepted and assumed that the era of cheap and easily accessible fossil fuel is coming to its end. Thus, the production of biodiesel from renewable raw materials has become very important in recent years as a potential alternative to partially satisfy the future energy demands in the transport sector [1,2]. In this respect, transesterification is currently the most attractive and widely accepted methodology used for biodiesel production [3]. This usually involves the use of homogeneous base catalysts operating under mild * Correspondence: qo1lumad@uco.es 1 Department of Organic Chemistry, University of Cordoba, Campus de Rabanales, Bldg. Marie Curie, 14014, Cordoba, Spain 4 Seneca Green Catalyst S.A., Bldg Centauro, Technological Science Park of Cordoba, Rabanales XXI, 14014, Córdoba, Spain Full list of author information is available at the end of the article conditions. In order to shift the equilibrium towards the production of fatty acid methyl esters (FAME), an excess of methanol is normally utilized in the process to produce biodiesel. However, glycerol is always produced as a contaminant in addition to alkaline impurities that need to be removed. The glycerol by-product is the main drawback of this method because it lowers the overall efficacy of the process and its removal requires several consecutive water washing steps and hence creates a demand for a lot of water [4,5]. A series of alternative methods are being investigated to avoid the problems associated with the generation of glycerol in the conventional process. They all are based on achieving various glycerol derivatives in the same transesterification process. In this way, the complex and expensive additional separation process of glycerol is eliminated and the overall yield of the process increased. These novel methodologies are able to prepare methyl 2014 Luna et al.; licensee Springer. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
2 Luna et al. Bioresources and Bioprocessing 2014, 1:11 Page 2 of 13 esters of fatty acids from lipids using different acyl acceptors instead of methanol in the transesterification process that result in glycerol derivatives as co-products [6]. For example, the transesterification reaction of triglycerides with dimethyl carbonate (DMC) [7], ethyl acetate [8], or methyl acetate [9] generates a mixture of three molecules of FAME or fatty acid ethyl esters (FAEE) and one of glycerol carbonate (GC) or glycerol triacetate (triacetin). These mixtures, including the glycerol derivative molecules, have physicochemical properties similar to biodiesellike biofuel [10]. In the present case, the atom efficiency is also improved because the total number of atoms involved in the reaction is obtained in the final mixture. On the other hand, we have recently developed a protocol for the preparation of a new biodiesel-like biofuel, which integrates glycerol into monoglycerides via 1,3- regiospecific enzymatic transesterification of sunflower oil using free [11] and immobilized [12-14] porcine pancreatic lipase (PPL). The operating conditions of such enzymatic process were more efficient compared to the preparation method of conventional biodiesel and did not generate any acidic or alkaline impurities. Thus, the Ecodiesel biofuel [12-15] obtained through the partial ethanolysis of triglycerides with 1,3-selective lipases is constituted by a mixture of two parts of FAEE and one of monoacylglyceride (MG). These glycerol derivative MGs are soluble components in the FAEE mixture suitable for use as a biodiesel-like biofuel. In the current case, ethanol was used as a cheap reagent, instead of the more expensive substrates such as dimethyl carbonate or methyl acetate. This procedure takes advantage of the 1,3-selective nature of the most known lipases, which allows to terminate the process in the second step of the alcoholysis to yield the mixture of 2 mol of FAEE and 1 mol of MG as products (Figure 1). In this way, the glycerol is kept in the form of monoglyceride, which avoids the production of glycerol as by-product, reducing the environmental impact of the process. In summary, the enzymatic process to obtain this biodiesel-like biofuel operates under much smoother conditions, impurities are not produced, and the biofuel produced exhibits similar physicochemical properties to those of conventional biodiesel. Last but not least, MGs enhance biodiesel lubricity, as it was demonstrated by recent studies [16-18]. Besides, the ethanol that is not spent in the enzymatic process remains in the reaction mixture, and the blend obtained after the reaction can be used directly as fuel. In this respect, very recent studies [19-21] have shown that blends of diesel fuel and biodiesel containing ethanol reduce power output slightly than regular diesel. No significant difference in the emissions of CO 2, CO, and NO x between regular diesel and biodiesel or ethanol and diesel blends was observed. Furthermore, the use of these blends resulted in a reduction of particulate matter. Consequently, such blends can be used in a diesel engine without any modification despite the slightly reduced power output compared to pure diesel. Thus, the Ecodiesel is currently utilized as a blend of fatty acid alkyl esters with ethanol, alone or with any proportion of diesel fuel [21,22]. The high cost of traditional industrial lipases restricted their use in biofuel production, but the current availability of the recombinant purified in sufficiently high quantities has helped to achieve the economic viability as the crucial factors affecting productivity of enzymatic biodiesel synthesis are the suitable raw materials and the selected lipase. The stability and catalytic efficiency of the latter can be improved by optimizing reaction conditions such as substrate concentrations, temperature, water activity, and alkaline concentration of the enzyme's microenvironment [23]. In this respect, although Ecodiesel was initially obtained using pig pancreatic lipases (PPL), remarkable results have been also obtained with a low-cost purified microbial lipase, Lipopan50BG(NovozymesA/S,Bagsværd,Denmark) [15], from Thermomyces lanuginosus microorganism, usually used as bread emulsifier (bread improver) [24]. To our knowledge, this lipase has not been described as a biocatalyst in any chemical process. The application of an available lipase on an industrial scale is a significant Figure 1 Representative scheme of Ecodiesel production by application of 1,3-selective enzymatic catalysis.
3 Luna et al. Bioresources and Bioprocessing 2014, 1:11 Page 3 of 13 approximation to get an economically feasible biofuel production by enzymatic method. However, this lipase has a main drawback: it cannot be reused, since the purified lipase extract is meant to be in a soluble form. TheaimofthepresentstudyistoevaluateNovozym 435, a commercial lipase from Candida antarctica, recombinant (CALB), expressed in Aspergillus niger and immobilized onto an acrylic macroporous resin [25]. Novozym 435 has previously been used in the synthesis of conventional biodiesel as well as in other transesterification processes such as interesterification [26] and in lipase-catalyzed biodiesel production by isopropanolysis of soybean oil [27]. In this respect, Novozym 435 is also described very recently in lipase-catalyzed simultaneous biosynthesis of biodiesel and glycerol carbonate, by using dimethyl carbonate as acyl acceptor to obtain a biodiesel-like biofuel without glycerol generation [28]. Thus, in this study, it is intended to put in value the 1,3-selective behavior of these commercial lipases to make feasible the profitable production of alternative biofuels [5], using an enzymatic approach. Methods In this respect, in order to evaluate the influence of crucial parameters (lipase amount, temperature, oil/ethanol volumetric relationship, and alkaline environment) in the transesterification reaction, a multifactorial design of experiments and response surface methodology (RSM) using a multifactorial design of experiments with three factors run by the software Stat Graphics version XV.I were used. This study has been developed to optimize the catalytic behavior of this 1,3-selective lipase (N435) in the partial ethanolysis of sunflower oil, to obtain a biofuel that integrates glycerol as MG together with the different FAEEs in the enzymatic ethanolysis process as well as with the excess of unreacted ethanol. This biofuel mixture currently named Ecodiesel is able to directly operate diesel engines, alone or in whichever mixture with diesel fuel, without anymore separation or purification process. Commercial sunflower oil was locally obtained. The chromatographically pure ethyl esters of palmitic acid, stearic acid, oleic acid, linoleic acid, and linolenic acid were commercially obtained from AccuStandard (New Haven, CT, USA), and hexadecane (cetane) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals like absolute ethanol and sodium hydroxide were pure analytical compounds (99.5%) obtained commercially from Panreac (Castellar Del Valles, Spain). Novozym 435, the lipase B from C. antarctica (CALB), was kindly provided by Novozymes A/S. This commercial lipase is produced by submerged fermentation of a genetically modified A. niger microorganism and adsorbed on a macroporous acrylic resin. The specific activity of this commercial lipase in the hydrolysis of tributyrin was 3,000 U/g. In this respect, additional experiments were conducted with a pure lipase B from C. antarctica, recombinant from Aspergillus oryzae, powder, from Sigma-Aldrich. The specific activity of this commercial lipase in the hydrolysis of tributyrin was >1,000 U/g. In this way, the existence of possible differences in the catalytic behavior of CALB lipases by the influence of the support could be detected. Results and discussion Comparative chromatograms of standardized reaction products To identify the most characteristic components of biofuels obtained by enzymatic alcoholysis, as well as to compare their rheological properties, several commercial standards of reference for FAME, FAEE, MG, and triacylglycerol (TG) were used, as shown in Figure 2. Here, a representative sample of monoglycerides of sunflower oil is also included, which was easily achieved by the substitution of methanol or ethanol by glycerol, in a conventional alcoholysis process with KOH as homogeneous catalyst following standard experimental conditions. Here, we can see that the different esters of fatty acids (FAEs), which compose the lipid profile of the sunflower oil, display retention times (RTs) slightly higher than those of cetane (n-hexane) used as internal standard. Thus, whereas RT of cetane is around 10 min, all RTs of FAEs appear in the range of 16 to 26 min. These are composed of methyl, ethyl, and glycerol esters (the latter constitute MGs) of palmitic, stearic, linoleic, and oleic acids. Thus, palmitic acid (C16:0) derivatives are grouped in a narrow range of RT, 16 to 17 min. Derivatives of oleic (C18:1) and linoleic acid (C18:2) are grouped in RT of 19 to 21 min, with the exception of glycerol ester of oleic acid, or what is the same, the MG of the oleic acid has a different behavior, with a RT = 26 min. Glycerol RT appears at 5 min, before cetane. The absence of this compound in the obtained chromatograms clearly demonstrates the selective nature of the studied enzymatic transesterification reaction. In Figure 2, the presence of diacylglycerol (DG) with higher retention times, 40 to 60 min, can also be seen, which do not allow its integration into the GC chromatogram, so that it is necessary to determine DG together and TG by using an internal standard such as cetane here employed. It should be noted that the differences in RT values between MG and DG are much higher than those existing between MG and FAME or FAEE, such as it is expected by the differences between their corresponding molecular weights. At the same time, it is clear that the FAMEs, FAEEs, and MGs display somewhat higher RT values than cetane, but within the molecular weight range, which allows considering similar chemical-physical properties between the FAE and the hydrocarbons that constitute diesel.
4 Luna et al. Bioresources and Bioprocessing 2014, 1:11 Page 4 of 13 Retention Time (RT/min) Peak 0-7 Solvent (ethanol + dichloromethane 50:50) 5 Glycerol 9-10 Internal Standard (n-hexadecane/ Cetane) 16 Palmitic FAME (16:0) 17 Palmitic MG (16:0) 18 Palmitic FAEE (16:0) 19 Estearic (18:0), Linoleic (18:2), Oleic (18:1) FAME 20 Estearic (18:0), Linoleic (18:2), Oleic (18:1) FAEE Estearic (18:0), Linoleic (18:2) MG 26 Oleic (18:1) MG DG + TG Figure 2 Superimposed chromatograms of sunflower oil, FAME, FAEE, and MG. Since the retention times of different derivatives of fatty acids are considered very closely related to the chemicalphysical properties of these compounds, the great similarity of RT values obtained is a clear demonstration of the similarity among the rheological properties of the different MGs with their corresponding FAMEs or FAEEs, which are crucial to allow its use as fuel able to substitute for petroleum products. Consequently, conversion is a reaction parameter where all molecules (FAEE, MG, and DG) obtained in the ethanolysis of TG are included (as %); it will be considered as a very different parameter, with respect to selectivity, where FAEEs and MGs are only included (as %), all of them having RT values lower than 26 min. These molecules exhibit RT values similar to hydrocarbons present in conventional diesel, so that they all could exhibit similar physicochemical and rheological properties. However, a high conversion, even 100%, could contain a high proportion of DG molecules, with high molecular weight and high viscosity values. Consequently, a very high selectivity, indicating a very high percentage of FAEEs and MGs, could promote a viscosity value close to the petroleum diesel, so that the highest conversion value is not enough a guaranty of lower viscosity values. Thus, both parameters will be provided as GC analysis results of reaction products. Taking into account that retention times of a complex mixture of hydrocarbons constituting fossil diesel fuel are ranging from 1 to 25 min, it is used as a reference value for different biofuels (FAME, FAEE, MG) as selectivity value, all those FAEs that present RT values coincident with the hydrocarbons constituting diesel, or those with RT lower than 25 min, as it is expected that they also present similar physicochemical and rheological properties to the conventional diesel.
5 Luna et al. Bioresources and Bioprocessing 2014, 1:11 Page 5 of 13 Analysis of variance and optimization of the reaction parameters by RSM Theanalysisofvariancemethodshasbecomeveryattractive in reaction parameter optimization and in the evaluation of the effects of the parameters in the TG transesterification reaction [9,15,27] due to their effectiveness in the analysis of variables. Thus, results obtained operating under the 36 runs - each one with different experimental conditions, selected by the multifactorial design of experiments with three factors, developed by the software Stat Graphics version XV.I., where two of them are developed at three levels and the other at two levels, indicated in Table 1 - are shown in Table 2. The quantity of supported lipase (N435) in all these experiments was fixed to 0.5 g. All experiments were duplicated in order to avoid experimental errors. Data was fitted to a quadratic polynomial model using the software. The quadratic polynomial model was highly significant and sufficient to explain the relationship between conversion/selectivity/kinematic viscosity and important experimental variables. Thus, the results of factorial design suggested that the major factors affecting the transesterification, for the production of biofuels integrating glycerol as monoacylglycerols, were temperature and oil/ethanol ratio (v/v). The values of correlation coefficients, R 2, were for conversion, for selectivity, and for kinematic viscosity, respectively, which imply a good fit between models and experimental data in Pareto graphics, with respect to conversion, selectivity, and viscosity, as indicated in Figure 3a. The adjusted correlation coefficients R 2 were 0.762, 0.889, and for conversion, selectivity, and kinematic viscosity, respectively. Obtained results pointed out that the temperature and oil/ethanol (v/v) ratios were also important parameters influencing the conversion and viscosity in the systems (p < 0.05). The software also allows to obtain equations (Equations 1, 2, and 3), remarkably simpler as compared to initial ones after the elimination of non-influent parameters in the model for conversion, selectivity, and kinematic viscosity. These equations describe the model created and give solutions for the dependent variable based on the independent variable combinations, selecting the most significant in the response. Thus, taking into account that R is the oil/ethanol Table 1 Process parameters in factorial design: coded and actual values Variables Unit Levels Temperature C Oil/ethanol ratio (v/v) ml/ml 12/1,75-12/3.5 Alkaline environment μl (NaOH 10 N) 8 (12.5) 10 (5) 12 (50) ratio (v/v), alkaline environment is obtained by the addition of different microliters of NaOH 10 N, and T is the reaction temperature, ½Conversion ð% Þ ¼ 93:57 þ 5:06 T þ 7:82 R 6:42 T 2 6:53 T R 3:25 R ph ð1þ ½Selectivity ð% Þ ¼ 35:97 þ 6:26 T 2:25 ph þ4:50 R þ 6:93 T ph 12:22 T R þ 6:13 ph 2 6:61 ph R ð2þ ½ViscosityðcStÞ ¼ 17 :27 þ 1:65 T 2:32 R þ0:97 T 2 þ 1:95 T R þ1:20 R ph : ð3þ The surface plots in Figure 3b, described by the regression model, were drawn to display the effects of the independent variables on conversion, selectivity, and kinematic viscosity. Here, the influence of the different variables in the conversion of the systems can be clearly seen. This model showed that the optimum values for the parameters to maximize transesterification yield (conversion, selectivity, kinematic viscosity) were intermediate temperatures (30 C), maximum amount of aqueous NaOH 10 N added (50 μl), and the maximum oil/ethanol (v/v) ratio=6:1 studied. Conversions up to 100%, selectivities around 70%, and values of kinematic viscosity about 10 to 15 mm 2 s 1 could be achieved under these conditions, which in theory will render feasible the utilization of the obtained biofuel in blends with diesel. For example, by the addition of only 35% of diesel fossil, to this biofuel, a viscosity reduction at 4.8 mm 2 s 1, a value within the acceptance limits of the EN 14214, is obtained. Effect of the amount of lipase The effect of the amount of lipase in the reaction media is very important to select the correct reaction conditions. This parameter was evaluated in order to choose the necessary amount of lipase that maximizes yield without mass transfer limitations. Previous optimized values obtained from RSM (12 ml oil/3.5 ml EtOH, 30 C, 12.5 μl NaOH 10 N, stirring speed higher than 300 rpm for 2 h) were chosen. We can see in Figure 4 how the reaction yield and viscosity were improved, so better values could be obtained using more quantities of biocatalyst, but larger amounts of enzymes will have a detrimental effect on the economics of the process.
6 Luna et al. Bioresources and Bioprocessing 2014, 1:11 Page 6 of 13 Table 2 Experiment matrix of factorial design and the response obtained for conversion, selectivity, and viscosity Run Parameters Temperature Oil/ethanol ratio ph Conversion (%) Selectivity (%) Kinematic viscosity (mm 2 s 1 ) Repeated experiments Study of enzyme activity in successive reactions Since we have been doing these successive reactions, the reaction conditions have been changed such that the procedure enables the study according to OVAT ( variable at one time ) methodology in which initial conditions are set and variables to study have been changed one by one. This method does not provide information on the combined effect of various reaction parameters, but it is useful to determine the influence of isolated variables. Because our research group had previous information about other lipase enzymes [12-15] and what were the most important reaction variables, it was decided to use this experimental
7 Luna et al. Bioresources and Bioprocessing 2014, 1:11 Page 7 of 13 (a) (b) (a.1) (b.1) (a.2) (b.2) (a.3) (b.3) Figure 3 Pareto graphics and response surface plots. (a) Pareto graphics: conversion (a.1), selectivity (a.2), and viscosity (a.3). (b) Response surface plot of more influential parameters: conversion (b.1), selectivity (b.2), and viscosity (b.3). methodology in order to evaluate in more detail the influence of those variables in the selected intervals for each study. In this way, reaction tests of N435 lipase systems have been carried out under the optimum conditions (alkaline environments, temperatures, and relative oil/ethanol ratio) previously determined by the RSM studies. These experimental conditions were similar to those previously obtained with T. lanuginosus, Lipopan 50 BG Lipopan [15]. In this respect, Table 3 shows a collection of the achieved
8 Luna et al. Bioresources and Bioprocessing 2014, 1:11 Page 8 of 13 Visc (cst) Yield (%) Graphic 2 0,05 0,1 0,2 0,3 0,4 0,5 Lipase Weight (g) 0 Graphic 1 0,05 0,1 0,2 0,3 0,4 0,5 Lipase Weight (g) Visc Sel Conv Figure 4 Influence of lipase amount of supported CALB in the performance of ethanolysis reactions, developed under standard conditions. Conversion and selectivity (graphic 1) and kinematic viscosity in cst (graphic 2). results in the transesterification reaction with this biocatalyst. Thus, in addition to obtain specific information about the influence of certain parameters (alkaline environment, temperature, etc.) on the behavior of the N435, its ability to be reused could be checked. In this sense, from the conversion, given that we start from 0.01 mol of TG, with 2 h as reaction time and 0.5 g of catalyst system (lipase CALB immobilized on acrylic polymer), we can calculate the transformation enzymatic capacity: turnover frequency (TOF number) expressed in micromoles of TG transformed per minute and per gram of catalyst (supported lipase). In order to be able to obtain the enzyme activity of the immobilized CALB, some research experiments with a purified powdered commercial lipase B from C. antarctica, recombinant from A. oryzae, have been conducted. Thus, operating under identical experimental conditions with those used in experiments carried out with N435, but using only 0.01 g of purified CALB in free form, a conversion of 50.6% was obtained. Excluding the possible influence of support effects in the lipase activity, and taking into account that 0.5 g of CALB immobilized enzyme produced a 47.7% conversion, it can be inferred that N435 has a catalytic activity corresponding to the 1.9% of the obtained catalytic activity bythefreecalb. Therefore, to obtain a reference value, and assuming that the activity of the immobilized enzyme is similar to that of the free enzyme, it can be considered that the N435 may contain about 1.9 wt% of immobilized enzyme. In any case, it works with this equivalent enzyme Table 3 Achieved results in the transesterification reaction with biocatalyst Run number NaOH amount Temperature EtOH/oil Viscosity Selectivity Conversion TOF Enzyme activity /12 (6:1) /12 (6:1) /12 (6:1) /12 (6:1) /12 (6:1) /12 (6:1) /12 (6:1) /12 (6:1) /12 (6:1) /12 (3:1) 18,8 17,9 27, /12 (6:1) /12 (4:1) /12 (5:1) /12 (6:1) /12 (7:1) /12 (8:1) Viscosity (cst), conversion (%), selectivity (%), turnover frequencies (TOF in μmol min 1 g cat 1 ), and enzyme activities (U/mg lipase) of the obtained biodiesel by using the same biocatalyst (0.5 g of N435) in successive reactions under the evaluated different experimental conditions: temperature ( C), NaOH amount (μl of NaOH 10 N solution), and EtOH/oil ratio (ml/ml or mol/mol).
9 Luna et al. Bioresources and Bioprocessing 2014, 1:11 Page 9 of 13 activity. From this parameter, a reference value of the catalytic activity of the immobilized enzymes, expressed in milligrams of enzyme required to convert 1 μmol of TG per minute (U/mg), can also be calculated. If we subdivide the results in Table 3 in terms of the variable that has been changed each time in the study with OVAT methodology and if we organize these results in their corresponding graphics (Figure 5), the influence of each variable on the transesterification (a.2) (a) reaction yield is clearly displayed and we reached similar conclusions to those obtained in the analysis of variance (ANOVA) study by RSM; the optimum reaction parameters for the enzymatic ethanolysis reaction with lipase N435, CALB (C. antarctica lipase B) immobilized on acrylic resin, are 30 C and 12.5 μl of10nnaoh, with 1:6 oil/ethanol molar ratio. Also, we can obtain from the data in Table 3 that N435 may be reused repeatedly. Thus, 16 reactions were (b.2) (b) Visc25 (cst) Graphic 2 Visc (cst) Visc (cst) Graphic 2 Visc (cst) º 25º 30º 35º 40º 0 3:01 4:01 5:01 6:01 7:01 8:01 Temperature (ºC) Ethanol/Oil Molar Ratio (a.1) (b.1) Graphic 1 Graphic 1 Yield (%) º 25º 30º 35º 40º Sel Conv Yield (%) Sel Conv Temperature (ºC) Ethanol/Oil Molar Ratio Figure 5 Reaction temperature and oil/ethanol molar ratio. Influence of the (a) reaction temperature and (b) oil/ethanol molar ratio in the enzymatic ethanolysis reaction performance of supported CALB developed under standard conditions. Conversion and selectivity (graphic 1) and viscosity in cst (graphic 2).
10 Luna et al. Bioresources and Bioprocessing 2014, 1:11 Page 10 of 13 developed successively without an appreciable loss in catalytic activity in the ethanolysis reaction of sunflower oil, operating under different experimental conditions. However, an important drawback is associated to its acrylic polymeric character. Thus, to recover the immobilized lipase on macroporous resin, after each successive reaction, it is necessary to carry out the centrifugation of reaction products. Finally, this research, developed in order to improve a new methodology to integrate the glycerol as different monoacylglycerol molecules, is in connection with precedent researches performed to determine the optimal experimental conditions of the selective ethanolysis reaction. In this respect, the results here obtained resemble those described with the commercial PPL lipases [12-14] and with a low-cost purified lipase, [15] from T. lanuginosus, Lipopan 50 BG (Novozymes A/S), widely used in the bakery industry as an emulsifier [24]. In this respect, the possibility of application of commercial immobilized lipases in this process is now explored to exploit the opportunity of its reuse, thus lowering its operational cost and consequently increasing the economic potential of their industrial application. Thus, in this study, the behavior of a commercial supported lipase, Novozym 435, is determined because this may involve some technological advantages in its development, so that this study has the aim to prove its viability, its yield, and its possibility of reuse, what will mean an important advance in its biotechnological applications in the biofuel enzymatic production [5]. Currently, results obtained in this study indicate that this commercial supported lipase is specially efficient in obtaining 1,3-selective ethanolysis processes, where glycerol is maintained as MG in the biofuel mixture, with the different obtained FAEEs and together to the excess of unreacted ethanol. In this way, a new kind of biodiesel currently named Ecodiesel is achieved, constituted by a mixture of monoacylglycerols and FAEEs mainly (1/2 nominally), which can be used in different blends with diesel fuel, without anymore separation or purification process. This new biofuel can be obtained with the help of N435 at very short reaction times (1 to 2 h) and under soft reaction conditions. Besides, not only a higher atomic yield is achieved, with respect to the conventional biodiesel reaction (because no glycerol by-product is generated), but also a purification step of residual glycerol is not necessary, so it can be used directly after its production. Despite the good results obtained in the transesterification reaction of sunflower oil with ethanol to produce FAEE and MG, unfortunately, the main drawback of this commercial biocatalyst is the low stability of the organic polymeric little spheres which constitute the catalytic powder that easily disintegrates in the first ethanolysis reaction and becomes a gelatinous material which is very uncomfortable to work in the successive reuses, so that centrifugation of reaction products is necessary for catalyst recovery to avoid important losses of catalyst. This additional centrifugation step may constitute a major drawback for the application of the process on an industrial scale. Experimental Ethanolysis reactions These reactions were performed according to the experimental procedure previously described [12-15] to determine the optimal conditions for obtaining the selective ethanolysis reaction, such as alkaline environment, amount of lipase, the oil/ethanol molar ratio (v/v), and temperature. Thus, enzymatic assays are carried out with 9.4 g (12 ml, 0.01 mol) of commercial sunflower oil at controlled temperatures (20 C to 40 C) in a 25-ml round bottom flask. Reaction mixtures were stirred with a conventional magnetic stirrer at a stirring speed higher than 300 rpm to avoid mass transfer limitations, along a reaction time of 2 h. Variable oil/alcohol volume ratios at different alkaline environments and different quantities of lipase are studied. The different oil/ethanol ratios (v/v) are obtained by introducing absolute ethanol volumes in the range of 1.75 to 3.5 ml, and the influence of different amounts of lipase is studied in the range of 0.05 to 0.5 g. The influence of alkaline environment values was achieved by adding different volumes (12.5 to 50 μl) of 10 N NaOH aqueous solution. In this regard, a blank reaction in the presence of the highestquantityofsolutionofnaohwasperformedtorule out a potential contribution from the homogeneous NaOH catalyzed reaction. Less than 10% conversion of the starting material was obtained, so that a homogenous base catalysis contribution can be considered as negligible under the investigated conditions. In this respect, the NaOH solution, to operate as a transesterification catalyst, needs usually to be used under higher temperatures and under higher catalyst concentrations (1 to 2 wt%), enough to generate sodium methoxide or ethoxide, the true catalyst of the homogeneous transesterification reaction. Thus, the use of a very small concentration of NaOH works as a promoter of the process, through its effect on the lipase biocatalytic activity. Accordingly, NaOH ions are responsible for the ph environment that affects the enzyme yield, because depending on the ph, the enzymatic protein offers different structures, more or less effective in the biocatalytic action. All variables were studied and optimized according to a factorial experimental design and a response surface methodology. Analytical method Reaction products were monitored by capillary column gas chromatography, using a Varian 430-GC gas chromatograph
11 Luna et al. Bioresources and Bioprocessing 2014, 1:11 Page 11 of 13 (Varian Inc., Palo Alto, CA, USA), connected to a HT5 capillary column (25 m 0.32 mm ID 0.1 μm, SGE, Supelco) with a flame ionization detector (FID) at 450 C and splitless injection at 350 C. Helium is used as carrier gas with a flow of 1.5 ml/min. A heating ramp from 90 C to 200 C at a rate of 7 C/min has been applied, followed by another ramp from 200 C to 360 C at a rate of 15 C/ min, maintaining the temperature of the oven at 360 C for 10 min using as internal standard n-hexadecane (cetane) to quantify the content of ethyl esters and the different glycerides (mono-, di-, and triglycerides) with the help of several commercial standard fatty acid esters. This method allows us to make a complete analysis of the sample in a single injection and in a time not longer than 60 min, which simplifies the process and increases the speed of analysis [12-15]. Considering that sunflower oil is constituted by a mixture of fatty acids in variable proportion (mainly linoleic, oleic, palmitic, and stearic acids), the results obtained are expressed as the relative amounts of the corresponding ethyl esters (FAEE, fatty acid ethyl esters), monoglycerides (MG), and diglycerides (DG) that are integrated in the chromatogram. The amount of triglycerides (TG) which has not reacted is calculated from the difference to the internal standard (cetane). Thus, the conversion includes the total amount of triglyceride transformed (FAEE + MG + DG) in the ethanolysis process, and selectivity makes reference to the relative amount of FAEE + MG obtained. The latter are the ones having retention times close to the cetane standard, which is the reference hydrocarbon for diesel fuel. Viscosity measurements The transesterification reactions of oils and fats are basically carried out to obtain an important reduction in the viscosity of these materials, as they share similar values in all of other chemical-physical significant parameters with the fossil diesel except the viscosity. In this respect, most oils exhibit viscosities in the range of 30 to 45 mm 2 /s cst values, while the fossil diesel is in the range of 2.5 to 6 cst values. Thus, due to the importance of viscosity for the correct running of diesel engines, this parameter becomes a critical factor to change the chemical-physical properties of vegetable oils before their use as biofuel. The transesterification process of oils and fats is actually developed in order to obtain a noticeable lowering of viscosity in oils to employ the resulting product as biofuel in current existing diesel engines. Thus, accurate viscosity measurements are critical to assess the quality of biofuels produced, since unsuitable viscosity values can decisively affect the correct working conditions of the diesel engine. Therefore, the characterization of this parameter is essential to evaluate the result obtained in the process of ethanolysis. Viscosities were determined in a capillary viscometer Oswald Proton Cannon-Fenske Routine Viscometer 33200, size 150. This is based on determining the time needed for a given volume of fluid passing between two points marked on the instrument. The kinematic viscosity is given by the ratio between the dynamic viscosity (h, in Poise,g/cm s) and the density (r, ing/cm 3 ) υ = h/r (in cm 2 /s or centistokes (cst), mm 2 /s). Samples, previously centrifuged at 3,500 rpm for 10 min and filtered at 50 C, are immersed in a thermostatic bath at 40 C for 15 min, making sure that the temperature is stable. Then, samples are introduced into the viscometer and this, in turn, in the water bath, making sure that it is rigorously positioned vertically, with the bottom end at a minimum distance of 2 cm from the floor of the bath [12-15]. Experimental design The effect of process parameters in the enzymatic transesterification reaction to obtain the optimum conditions for the viscosity, selectivity, and conversion was studied using a multifactorial design of experiments with three factors run by the software Stat Graphics version XV.I. Two of them are developed at three levels and the last one at two levels, so that it gives 36 runs. The experiments were performed in random order. The experimental parameters selected for this study were reaction temperature, oil/ethanol ratio (v/v), and different alkaline environments obtained by the addition of variable volumes (in μl) of NaOH 10 N. Table 1 shows the coded and actual values of the process parameters used in the design matrix. Statistical analysis The experimental data obtained from experimental design were analyzed by response surface methodology (RSM) [9,15,27]. A mathematical model, following a second-order polynomial equation, was developed to describe the relationships between the predicted response variable (viscosity, conversion, and selectivity) and the independent variables of reaction conditions, as shown in the Equation 4, where Y is the predicted response variable; β 0, β i, β ii, and β ij are the intercept, linear, quadratic, and interaction constant coefficients of the model, respectively; and X i and X j (i =1, 3; j =1, 3; i j) represent the coded independent variables. Y ¼ β 0 þ X3 i¼1 β 0 x i þ X3 i¼1 β ii x 2 i þ XX3 i<j¼1 β ij x i x j ð4þ Response surface plots were developed using the fitted quadratic polynomial equation obtained from regression analysis, holding one of the independent variables at constant values corresponding to the stationary point and changing the order of two variables. The quality of
12 Luna et al. Bioresources and Bioprocessing 2014, 1:11 Page 12 of 13 the fit of the polynomial model equation was evaluated by the coefficient of determination R 2, and its regression coefficient significance was checked with F-test. Confirmatory experiments were carried out in order to validate the model, using combinations of independent variables which were not part of the original experimental design but within the experimental region. Conclusions Novozym 435 lipase was evaluated in the 1,3-selective ethanolysis of sunflower oil to integrate the glycerol as monoacylglycerol molecules. On the basis of RSM analysis, the optimum conditions for synthesis were 1:6 molar oil/etoh ratio, 30 C, and 12.5 μl NaOH 10 N aqueous solutions, with stirring speed higher than 300 rpm for 2 h and 0.5 g of biocatalyst. Accordingly, its ability to be repeatedly reused could open a new way for the production of alternative biodiesel using an enzymatic approach, which is technically feasible and economically viable. The main drawback is the permanent need for centrifugation to recover the biocatalyst for the next reuse. Abbreviations ANOVA: analysis of variance; CALB: Candida antarctica lipase B; DG: diacylglycerol; FAE: fatty acid esters; FAEE: fatty acid ethyl esters; FAME: fatty acid methyl esters; MG: monoacylglycerol; OVAT: variable at one time; RSM: response surface methodology; TG: triacylglycerol; TOF: turnover frequency. Competing interests The authors declare that they have no competing interests. Authors' contributions CL, CV, EDS, DL, JC, AP, FMB, and AAR have made substantive intellectual contributions to this study, making substantial contributions to the conception and design of it as well as to the acquisition, analysis, and interpretation of data. All of them have been also involved in the drafting and revision of the manuscript. All authors read and approved the final manuscript. Acknowledgements Grants from the Spanish Ministry of Economy and Competitiveness (Project ENE ), Spanish Ministry of Education and Science (Projects CTQ and CTQ C02-02), FEDER funds and Junta de Andalucía FQM 0191, PO8-RMN and P11-TEP-7723 are gratefully acknowledged by the authors. We are also grateful to Novozymes A/S, Denmark, for the kind supply of the macroporous resin immobilized lipase from Candida antarctica (Novozym 435). Author details 1 Department of Organic Chemistry, University of Cordoba, Campus de Rabanales, Bldg. Marie Curie, 14014, Cordoba, Spain. 2 Crystallographic Studies Laboratory, Andalusian Institute of Earth Sciences, CSIC, Avda. Las Palmeras, n 4, 18100, Armilla, Granada, Spain. 3 Department of Microbiology, University of Cordoba, Campus de Rabanales, Ed. Severo Ochoa, 14014, Cordoba, Spain. 4 Seneca Green Catalyst S.A., Bldg Centauro, Technological Science Park of Cordoba, Rabanales XXI, 14014, Córdoba, Spain. Received: 22 April 2014 Accepted: 22 July 2014 Published: 31 August 2014 References 1. Demirbas A (2009) Political, economic and environmental impacts of biofuels: a review. Appl Energy 86: Luque R, Herrero-Davila L, Campelo JM, Clark JH, Hidalgo JM, Luna D, Marinas JM, Romero AA (2008) Biofuels: a technological perspective. Energy Environ Sci 1: Oh PP, Lau HLN, Chen JH, Chong MF, Choo YM (2012) A review on conventional technologies and emerging process intensification (PI) methods for biodiesel production. Renew Sust Energy Rev 16: Saleh J, Dube MA, Tremblay AY (2011) Separation of glycerol from FAME using ceramic membranes. Fuel Process Technol 92: Calero J, Luna D, Sancho ED, Luna C, Posadillo A, Bautista FM, Romero AA, Berbel J, Verdugo C (2014) Technological challenges for the production of biodiesel in arid lands. J Arid Environ 102: Ganesan D, Rajendran A, Thangavelu V (2009) An overview on the recent advances in the transesterification of vegetable oils for biodiesel production using chemical and biocatalysts. Rev Environ Sci Biotech 8: Ilham Z, Saka S (2010) Two-step supercritical dimethyl carbonate method for biodiesel production from Jatropha curcas oil. Bioresour Technol 101: Kim SJ, Jung SM, Park YC, Park K (2007) Lipase catalyzed transesterification of soybean oil using ethyl acetate, an alternative acyl acceptor. Biotechnol Bioprocess Eng 12: Tan KT, Lee KT, Mohamed AR (2011) A glycerol-free process to produce biodiesel by supercritical methyl acetate technology: an optimization study via response surface methodology. Bioresour Technol 102: Casas A, Ruiz JR, Ramos MJ, Perez A (2010) Effects of triacetin on biodiesel quality. Energy Fuels 24: Verdugo C, Luque R, Luna D, Hidalgo JM, Posadillo A, Sancho ED, Rodriguez S, Ferreira-Dias S, Bautista F, Romero AA (2010) A comprehensive study of reaction parameters in the enzymatic production of novel biofuels integrating glycerol into their composition. Bioresour Technol 101: Caballero V, Bautista FM, Campelo JM, Luna D, Marinas JM, Romero AA, Hidalgo JM, Luque R, Macario A, Giordano G (2009) Sustainable preparation of a novel glycerol-free biofuel by using pig pancreatic lipase: partial 1,3- regiospecific alcoholysis of sunflower oil. Process Biochem 44: Luna D, Posadillo A, Caballero V, Verdugo C, Bautista FM, Romero AA, Sancho ED, Luna C, Calero J (2012) New biofuel integrating glycerol into its composition through the use of covalent immobilized pig pancreatic lipase. Int J Mol Sci 13: Luna C, Sancho E, Luna D, Caballero V, Calero J, Posadillo A, Verdugo C, Bautista FM, Romero AA (2013) Biofuel that keeps glycerol as monoglyceride by 1,3-selective ethanolysis with pig pancreatic lipase covalently immobilized on AlPO 4 support. Energies 6: Verdugo C, Luna D, Posadillo A, Sancho ED, Rodriguez S, Bautista F, Luque R, Marinas JM, Romero AA (2011) Production of a new second generation biodiesel with a low cost lipase derived from Thermomyces lanuginosus: optimization by response surface methodology. Catal Today 167: Xu YF, Wang QJ, Hu XG, Li C, Zhu XF (2010) Characterization of the lubricity of bio-oil/diesel fuel blends by high frequency reciprocating test rig. Energy 35: Haseeb A, Sia SY, Fazal MA, Masjuki HH (2010) Effect of temperature on tribological properties of palm biodiesel. Energy 35: Wadumesthrige K, Ara M, Salley SO, Ng KYS (2009) Investigation of lubricity characteristics of biodiesel in petroleum and synthetic fuel. Energy Fuels 23: Çelikten I (2011) The effect of biodiesel, ethanol and diesel fuel blends on the performance and exhaust emissions in a diesel engine. GU J Sci 24: Cheenkachorn K, Fungtammasan B (2009) Biodiesel as an additive for diesohol. Int J Green Energy 6: Jaganjac M, Prah IO, Cipak A, Cindric M, Mrakovcic L, Tatzber F, Ilincic P, Rukavina V, Spehar B, Vukovic JP, Telen S, Uchida K, Lulic Z, Zarkovic N (2012) Effects of bioreactive acrolein from automotive exhaust gases on human cells in vitro. Environ Toxicol 27: Pang XB, Mu YJ, Yuan J, He H (2008) Carbonyls emission from ethanol-blended gasoline and biodiesel-ethanol-diesel used in engines. Atmos Environ 42: Szczesna-Antczak M, Kubiak A, Antczak T, Bielecki S (2009) Enzymatic biodiesel synthesis - key factors affecting efficiency of the process. Renew Energy 34: Moayedallaie S, Mirzaei M, Paterson J (2010) Bread improvers: comparison of a range of lipases with a traditional emulsifier. Food Chem 122: Xu Y, Nordblad M, Woodley JM (2012) A two-stage enzymatic ethanol-based biodiesel production in a packed bed reactor. J Biotechnol 162:
13 Luna et al. Bioresources and Bioprocessing 2014, 1:11 Page 13 of Yara-Varon E, Joli JE, Torres M, Sala N, Villorbina G, Mendez JJ, Canela- Garayoa R (2012) Solvent-free biocatalytic interesterification of acrylate derivatives. Catal Today 196: Chang C, Chen JH, Chang CMJ, Wu TT, Shieh CJ (2009) Optimization of lipase-catalyzed biodiesel by isopropanolysis in a continuous packed-bed reactor using response surface methodology. N Biotechnol 26: Min JY, Lee EY (2011) Lipase-catalyzed simultaneous biosynthesis of biodiesel and glycerol carbonate from corn oil in dimethyl carbonate. Biotechnol Lett 33: doi: /s y Cite this article as: Luna et al.: Production of a biodiesel-like biofuel without glycerol generation, by using Novozym 435, an immobilized Candida antarctica lipase. Bioresources and Bioprocessing :11. Submit your manuscript to a journal and benefit from: 7 Convenient online submission 7 Rigorous peer review 7 Immediate publication on acceptance 7 Open access: articles freely available online 7 High visibility within the field 7 Retaining the copyright to your article Submit your next manuscript at 7 springeropen.com
A Biofuel Similar to Biodiesel Obtained by Using a Lipase from Rhizopus oryzae, Optimized by Response Surface Methodology
 Energies 2014, 7, 3383-3399; doi:10.3390/en7053383 Article OPEN ACCESS energies ISSN 1996-1073 www.mdpi.com/journal/energies A Biofuel Similar to Biodiesel Obtained by Using a Lipase from Rhizopus oryzae,
Energies 2014, 7, 3383-3399; doi:10.3390/en7053383 Article OPEN ACCESS energies ISSN 1996-1073 www.mdpi.com/journal/energies A Biofuel Similar to Biodiesel Obtained by Using a Lipase from Rhizopus oryzae,
TECHNOLOGICAL CHALLENGES FOR THE PRODUCTION OF BIODIESEL IN ARID LANDS
 www.senecagreen.com Universidad de Córdoba BIODIVERSITY FOR BIOFUELS AND BIODIESEL IN ARID LANDS (BIO3) TECHNOLOGICAL CHALLENGES FOR THE PRODUCTION OF BIODIESEL IN ARID LANDS Diego Luna Departamento de
www.senecagreen.com Universidad de Córdoba BIODIVERSITY FOR BIOFUELS AND BIODIESEL IN ARID LANDS (BIO3) TECHNOLOGICAL CHALLENGES FOR THE PRODUCTION OF BIODIESEL IN ARID LANDS Diego Luna Departamento de
Biofuel that Keeps Glycerol as Monoglyceride by 1,3-Selective Ethanolysis with Pig Pancreatic Lipase Covalently Immobilized on AlPO 4 Support
 Energies 2013, 6, 3879-3900; doi:10.3390/en6083879 Article OPEN ACCESS energies ISSN 1996-1073 www.mdpi.com/journal/energies Biofuel that Keeps Glycerol as Monoglyceride by 1,3-Selective Ethanolysis with
Energies 2013, 6, 3879-3900; doi:10.3390/en6083879 Article OPEN ACCESS energies ISSN 1996-1073 www.mdpi.com/journal/energies Biofuel that Keeps Glycerol as Monoglyceride by 1,3-Selective Ethanolysis with
Biodiesel from soybean oil in supercritical methanol with co-solvent
 Available online at www.sciencedirect.com Energy Conversion and Management 49 (28) 98 912 www.elsevier.com/locate/enconman Biodiesel from soybean oil in supercritical methanol with co-solvent Jian-Zhong
Available online at www.sciencedirect.com Energy Conversion and Management 49 (28) 98 912 www.elsevier.com/locate/enconman Biodiesel from soybean oil in supercritical methanol with co-solvent Jian-Zhong
Production of a Biofuel that Keeps the Glycerol as a Monoglyceride by Using Supported KF as Heterogeneous Catalyst
 Energies 2014, 7, 3764-3780; doi:10.3390/en7063764 Article OPEN ACCESS energies ISSN 1996-1073 www.mdpi.com/journal/energies Production of a Biofuel that Keeps the Glycerol as a Monoglyceride by Using
Energies 2014, 7, 3764-3780; doi:10.3390/en7063764 Article OPEN ACCESS energies ISSN 1996-1073 www.mdpi.com/journal/energies Production of a Biofuel that Keeps the Glycerol as a Monoglyceride by Using
Synthesis of biodiesel from palm oil with dimethyl carbonate and methanol as reagent variation using KOH and enzyme catalyst
 IOP Conference Series: Earth and Environmental Science PAPER OPEN ACCESS Synthesis of biodiesel from palm oil with dimethyl carbonate and methanol as reagent variation using KOH and enzyme catalyst To
IOP Conference Series: Earth and Environmental Science PAPER OPEN ACCESS Synthesis of biodiesel from palm oil with dimethyl carbonate and methanol as reagent variation using KOH and enzyme catalyst To
Phase Distribution of Ethanol, and Water in Ethyl Esters at K and K
 Phase Distribution of Ethanol, and Water in Ethyl Esters at 298.15 K and 333.15 K Luis A. Follegatti Romero, F. R. M. Batista, M. Lanza, E.A.C. Batista, and Antonio J.A. Meirelles a ExTrAE Laboratory of
Phase Distribution of Ethanol, and Water in Ethyl Esters at 298.15 K and 333.15 K Luis A. Follegatti Romero, F. R. M. Batista, M. Lanza, E.A.C. Batista, and Antonio J.A. Meirelles a ExTrAE Laboratory of
Free and Total Glycerol in B100 Biodiesel by Gas Chromatography According to Methods EN and ASTM D6584
 Free and Total Glycerol in B100 Biodiesel by Gas Chromatography According to Methods EN 14105 and ASTM D6584 Introduction With today s increasing concern for the environment and the depletion of fossil
Free and Total Glycerol in B100 Biodiesel by Gas Chromatography According to Methods EN 14105 and ASTM D6584 Introduction With today s increasing concern for the environment and the depletion of fossil
Enzymatic Alholysis For Biodiesel Production From Waste Cooking Oil
 Enzymatic Alholysis For Biodiesel Production From Waste Cooking Oil R. Maceiras 1, A. Cancela*,1, M. Vega 2, M.C. Márquez 2 1 Chemical Engineering Department. University of Vigo. Campus Lagoas-Marcosende.
Enzymatic Alholysis For Biodiesel Production From Waste Cooking Oil R. Maceiras 1, A. Cancela*,1, M. Vega 2, M.C. Márquez 2 1 Chemical Engineering Department. University of Vigo. Campus Lagoas-Marcosende.
Project Reference No.: 40S_B_MTECH_007
 PRODUCTION OF BIODIESEL FROM DAIRY WASH WATER SCUM THROUGH HETEROGENEOUS CATALYST AND PERFORMANCE EVALUATION OF TBC DIESEL ENGINE FOR DIFFERENT DIESEL AND METHANOL BLEND RATIOS Project Reference No.: 40S_B_MTECH_007
PRODUCTION OF BIODIESEL FROM DAIRY WASH WATER SCUM THROUGH HETEROGENEOUS CATALYST AND PERFORMANCE EVALUATION OF TBC DIESEL ENGINE FOR DIFFERENT DIESEL AND METHANOL BLEND RATIOS Project Reference No.: 40S_B_MTECH_007
Application Note. Author. Introduction. Energy and Fuels
 Analysis of Free and Total Glycerol in B-100 Biodiesel Methyl Esters Using Agilent Select Biodiesel for Glycerides Application Note Energy and Fuels Author John Oostdijk Agilent Technologies, Inc. Introduction
Analysis of Free and Total Glycerol in B-100 Biodiesel Methyl Esters Using Agilent Select Biodiesel for Glycerides Application Note Energy and Fuels Author John Oostdijk Agilent Technologies, Inc. Introduction
Production of Biodiesel from Used Groundnut Oil from Bosso Market, Minna, Niger State, Nigeria
 Production of Biodiesel from Used Groundnut Oil from Bosso Market, Minna, Niger State, Nigeria Alabadan B.A. Department of Agricultural and Bioresources Engineering, Federal University, Oye Ekiti. Ajayi
Production of Biodiesel from Used Groundnut Oil from Bosso Market, Minna, Niger State, Nigeria Alabadan B.A. Department of Agricultural and Bioresources Engineering, Federal University, Oye Ekiti. Ajayi
COMPARISON OF TOTAL ENERGY CONSUMPTION NECESSARY FOR SUBCRITICAL AND SUBCRITICAL SYNTHESIS OF BIODIESEL. S. Glisic 1, 2*, D.
 COMPARISON OF TOTAL ENERGY CONSUMPTION NECESSARY FOR SUBCRITICAL AND SUBCRITICAL SYNTHESIS OF BIODIESEL S. Glisic 1, 2*, D. Skala 1, 2 1 Faculty of Technology and Metallurgy, University of Belgrade, Karnegijeva
COMPARISON OF TOTAL ENERGY CONSUMPTION NECESSARY FOR SUBCRITICAL AND SUBCRITICAL SYNTHESIS OF BIODIESEL S. Glisic 1, 2*, D. Skala 1, 2 1 Faculty of Technology and Metallurgy, University of Belgrade, Karnegijeva
Experimental Investigation and Modeling of Liquid-Liquid Equilibria in Biodiesel + Glycerol + Methanol
 11 2nd International Conference on Chemical Engineering and Applications IPCBEE vol. 23 (11) (11) IACSIT Press, Singapore Experimental Investigation and Modeling of Liquid-Liquid Equilibria in + + Methanol
11 2nd International Conference on Chemical Engineering and Applications IPCBEE vol. 23 (11) (11) IACSIT Press, Singapore Experimental Investigation and Modeling of Liquid-Liquid Equilibria in + + Methanol
Effects Of Free Fatty Acids, Water Content And Co- Solvent On Biodiesel Production By Supercritical Methanol Reaction
 Effects Of Free Fatty Acids, Water Content And Co- Solvent On Biodiesel Production By Supercritical Methanol Reaction Kok Tat Tan*, Keat Teong Lee, Abdul Rahman Mohamed School of Chemical Engineering,
Effects Of Free Fatty Acids, Water Content And Co- Solvent On Biodiesel Production By Supercritical Methanol Reaction Kok Tat Tan*, Keat Teong Lee, Abdul Rahman Mohamed School of Chemical Engineering,
Biodiesel from Various Vegetable Oils as the Lubricity Additive for Ultra Low Sulphur Diesel (ULSD)
 AMM-5 The 2 st Conference of Mechanical Engineering Network of Thailand 7-9 October 27, Chonburi, Thailand Biodiesel from Various Vegetable Oils as the Lubricity Additive for Ultra Low Sulphur (ULSD) Subongkoj
AMM-5 The 2 st Conference of Mechanical Engineering Network of Thailand 7-9 October 27, Chonburi, Thailand Biodiesel from Various Vegetable Oils as the Lubricity Additive for Ultra Low Sulphur (ULSD) Subongkoj
Conversion of Glycerol as By-Product from Biodiesel Production to Value-Added Glycerol Carbonate
 Conversion of as By-Product from Biodiesel Production to Value-Added Zul Ilham and Shiro Saka Abstract Current environmental issues, fluctuating fossil fuel price and energy security have led to an increase
Conversion of as By-Product from Biodiesel Production to Value-Added Zul Ilham and Shiro Saka Abstract Current environmental issues, fluctuating fossil fuel price and energy security have led to an increase
Keywords: Simarouba Glauca, Heterogeneous base catalyst, Ultrasonic Processor, Phytochemicals.
 PRODUCTION OF FATTY ACID METHYL ESTERS FROM SIMAROUBA OIL VIA ULTRASONIC IRRADIATION PROCESS, EFFECTIVE UTILIZATION OF BYPRODUCTS. TESTING AND EXTRACTION OF PHYTOCHEMICALS FROM SIMAROUBA OIL AND CAKE COLLEGE
PRODUCTION OF FATTY ACID METHYL ESTERS FROM SIMAROUBA OIL VIA ULTRASONIC IRRADIATION PROCESS, EFFECTIVE UTILIZATION OF BYPRODUCTS. TESTING AND EXTRACTION OF PHYTOCHEMICALS FROM SIMAROUBA OIL AND CAKE COLLEGE
New Biofuel Integrating Glycerol into Its Composition Through the Use of Covalent Immobilized Pig Pancreatic Lipase
 Int. J. Mol. Sci. 2012, 13, 10091-10112; doi:10.3390/ijms130810091 Article OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms New Biofuel Integrating Glycerol
Int. J. Mol. Sci. 2012, 13, 10091-10112; doi:10.3390/ijms130810091 Article OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms New Biofuel Integrating Glycerol
GC/MS Analysis of Trace Fatty Acid Methyl Esters (FAME) in Jet Fuel Using Energy Institute Method IP585
 GC/MS Analysis of Trace Fatty Acid Methyl Esters (FAME) in Jet Fuel Using Energy Institute Method IP585 Application Note Fuels Author James D. McCurry, Ph.D. Agilent Technologies, Inc. 850 Centerville
GC/MS Analysis of Trace Fatty Acid Methyl Esters (FAME) in Jet Fuel Using Energy Institute Method IP585 Application Note Fuels Author James D. McCurry, Ph.D. Agilent Technologies, Inc. 850 Centerville
Non-catalytic alcoholysis process for production of biodiesel fuel by using bubble column reactor
 Journal of Physics: Conference Series OPEN ACCESS Non-catalytic alcoholysis process for production of biodiesel fuel by using bubble column reactor To cite this article: S Hagiwara et al 2015 J. Phys.:
Journal of Physics: Conference Series OPEN ACCESS Non-catalytic alcoholysis process for production of biodiesel fuel by using bubble column reactor To cite this article: S Hagiwara et al 2015 J. Phys.:
This presentation focuses on Biodiesel, scientifically called FAME (Fatty Acid Methyl Ester); a fuel different in either perspective.
 Today, we know a huge variety of so-called alternative fuels which are usually regarded as biofuels, even though this is not always true. Alternative fuels can replace fossil fuels in existing combustion
Today, we know a huge variety of so-called alternative fuels which are usually regarded as biofuels, even though this is not always true. Alternative fuels can replace fossil fuels in existing combustion
Production of Biodiesel Fuel from Waste Soya bean Cooking Oil by Alkali Trans-esterification Process
 Current World Environment Vol. 11(1), 260-266 (2016) Production of Biodiesel Fuel from Waste Soya bean Cooking Oil by Alkali Trans-esterification Process Ajinkya Dipak Deshpande*, Pratiksinh Dilipsinh
Current World Environment Vol. 11(1), 260-266 (2016) Production of Biodiesel Fuel from Waste Soya bean Cooking Oil by Alkali Trans-esterification Process Ajinkya Dipak Deshpande*, Pratiksinh Dilipsinh
Optimization for Community Biodiesel Production from Waste Palm Oil via Two-Step Catalyzed Process
 Journal of Materials Science and Engineering A 5 (5-6) (2015) 238-244 doi: 10.17265/2161-6213/2015.5-6.008 D DAVID PUBLISHING Optimization for Community Biodiesel Production from Waste Palm Oil via Two-Step
Journal of Materials Science and Engineering A 5 (5-6) (2015) 238-244 doi: 10.17265/2161-6213/2015.5-6.008 D DAVID PUBLISHING Optimization for Community Biodiesel Production from Waste Palm Oil via Two-Step
SYNTHESIS OF BIODIESEL
 SYNTHESIS OF BIODIESEL AIM 1. To generate laboratory know-how for the process of production of biodiesel from the given oil feed stock 2. To perform basic mass and energy balance calculations for a large
SYNTHESIS OF BIODIESEL AIM 1. To generate laboratory know-how for the process of production of biodiesel from the given oil feed stock 2. To perform basic mass and energy balance calculations for a large
POLLUTION CONTROL AND INCREASING EFFICIENCY OF DIESEL ENGINE USING BIODIESEL
 POLLUTION CONTROL AND INCREASING EFFICIENCY OF DIESEL ENGINE USING BIODIESEL Deepu T 1, Pradeesh A.R. 2, Vishnu Viswanath K 3 1, 2, Asst. Professors, Dept. of Mechanical Engineering, Ammini College of
POLLUTION CONTROL AND INCREASING EFFICIENCY OF DIESEL ENGINE USING BIODIESEL Deepu T 1, Pradeesh A.R. 2, Vishnu Viswanath K 3 1, 2, Asst. Professors, Dept. of Mechanical Engineering, Ammini College of
Study of viscosity - temperature characteristics of rapeseed oil biodiesel and its blends
 Study of viscosity - temperature characteristics of rapeseed oil biodiesel and its blends Li Kong 1, Xiu Chen 1, a, Xiaoling Chen 1, Lei Zhong 1, Yongbin Lai 2 and Guang Wu 2 1 School of Chemical Engineering,
Study of viscosity - temperature characteristics of rapeseed oil biodiesel and its blends Li Kong 1, Xiu Chen 1, a, Xiaoling Chen 1, Lei Zhong 1, Yongbin Lai 2 and Guang Wu 2 1 School of Chemical Engineering,
BIODIESEL PRODUCTION BY A CONTINUOUS PROCESS USING A HETEROGENEOUS CATALYST
 J. Curr. Chem. Pharm. Sc.: 2(1), 2012, 12-16 ISSN 2277-2871 BIODIESEL PRODUCTION BY A CONTINUOUS PROCESS USING A HETEROGENEOUS CATALYST SHARDA D. NAGE *, K. S. KULKARNI, A. D. KULKARNI and NIRAJ S. TOPARE
J. Curr. Chem. Pharm. Sc.: 2(1), 2012, 12-16 ISSN 2277-2871 BIODIESEL PRODUCTION BY A CONTINUOUS PROCESS USING A HETEROGENEOUS CATALYST SHARDA D. NAGE *, K. S. KULKARNI, A. D. KULKARNI and NIRAJ S. TOPARE
Methanol recovery during transesterification of palm oil in a TiO2/Al2O3 membrane reactor: Experimental study and neural network modeling
 University of Malaya From the SelectedWorks of Abdul Aziz Abdul Raman 2010 Methanol recovery during transesterification of palm oil in a TiO2/Al2O3 membrane reactor: Experimental study and neural network
University of Malaya From the SelectedWorks of Abdul Aziz Abdul Raman 2010 Methanol recovery during transesterification of palm oil in a TiO2/Al2O3 membrane reactor: Experimental study and neural network
Optimized Method for Analysis of Commercial and Prepared Biodiesel using UltraPerformance Convergence Chromatography (UPC 2 )
 Optimized Method for Analysis of Commercial and Prepared Biodiesel using UltraPerformance Convergence Chromatography (UPC 2 ) Mehdi Ashraf-Khorassani, 1 Giorgis Isaac, 2 and Larry T. Taylor 1 1 Department
Optimized Method for Analysis of Commercial and Prepared Biodiesel using UltraPerformance Convergence Chromatography (UPC 2 ) Mehdi Ashraf-Khorassani, 1 Giorgis Isaac, 2 and Larry T. Taylor 1 1 Department
Kinetic Processes Simulation for Production of the Biodiesel with Using as Enzyme
 Kinetic Processes Simulation for Production of the Biodiesel with Using as Enzyme H.T.Hamd Abstract The esters components were produced by transesterification of the plant oil or for animal fat with methanol
Kinetic Processes Simulation for Production of the Biodiesel with Using as Enzyme H.T.Hamd Abstract The esters components were produced by transesterification of the plant oil or for animal fat with methanol
Determination of Free and Total Glycerin in Pure Biodiesel (B100) by GC in Compliance with EN 14105
 Application Note: 10215 Determination of Free and Total Glycerin in Pure Biodiesel (B100) by GC in Compliance with EN 14105 Fausto Munari, Daniela Cavagnino, Andrea Cadoppi, Thermo Fisher Scientific, Milan,
Application Note: 10215 Determination of Free and Total Glycerin in Pure Biodiesel (B100) by GC in Compliance with EN 14105 Fausto Munari, Daniela Cavagnino, Andrea Cadoppi, Thermo Fisher Scientific, Milan,
Cataldo De Blasio, Dr. Sc. (Tech.)
 Biodiesel Cataldo De Blasio, Dr. Sc. (Tech.) Aalto University, School of Engineering. Department of Mechanical Engineering. Laboratory of Energy Engineering and Environmental Protection. Sähkömiehentie
Biodiesel Cataldo De Blasio, Dr. Sc. (Tech.) Aalto University, School of Engineering. Department of Mechanical Engineering. Laboratory of Energy Engineering and Environmental Protection. Sähkömiehentie
KINETIC MODEL OF ALGAL BIODIESEL PRODUCTION UNDER SUPERCRITICAL METHANOLYSIS
 KINETIC MODEL OF ALGAL BIODIESEL PRODUCTION UNDER SUPERCRITICAL METHANOLYSIS Ashraf Amin, S. A. AboEl-Enin, G. El Diwani and S. Hawash Department of Chemical Engineering and Pilot Plant, National Research
KINETIC MODEL OF ALGAL BIODIESEL PRODUCTION UNDER SUPERCRITICAL METHANOLYSIS Ashraf Amin, S. A. AboEl-Enin, G. El Diwani and S. Hawash Department of Chemical Engineering and Pilot Plant, National Research
GC Analysis of Total Fatty Acid Methyl Esters (FAME) and Methyl Linolenate in Biodiesel Using the Revised EN14103:2011 Method
 GC Analysis of Total Fatty Acid Methyl Esters (FAME) and Methyl Linolenate in Biodiesel Using the Revised EN1413:211 Method Application Note Author James D. McCurry, Ph.D. Agilent Technologies Abstract
GC Analysis of Total Fatty Acid Methyl Esters (FAME) and Methyl Linolenate in Biodiesel Using the Revised EN1413:211 Method Application Note Author James D. McCurry, Ph.D. Agilent Technologies Abstract
BIODIESEL PRODUCTION USING SUPERCRITICAL ALCOHOLS AND DIFFERENT VEGETABLE OILS IN BATCH AND CONTINUOUS REACTORS
 BIODIESEL PRODUCTION USING SUPERCRITICAL ALCOHOLS AND DIFFERENT VEGETABLE OILS IN BATCH AND CONTINUOUS REACTORS P. Valle 1, A. Velez 2, P. Hegel 2, E.A. Brignole 2 * 1 LEC-ICEx DQ, Universidade Federal
BIODIESEL PRODUCTION USING SUPERCRITICAL ALCOHOLS AND DIFFERENT VEGETABLE OILS IN BATCH AND CONTINUOUS REACTORS P. Valle 1, A. Velez 2, P. Hegel 2, E.A. Brignole 2 * 1 LEC-ICEx DQ, Universidade Federal
OPTIMIZATION OF BIODIESEL PRODCUTION FROM TRANSESTERIFICATION OF WASTE COOKING OILS USING ALKALINE CATALYSTS
 OPTIMIZATION OF BIODIESEL PRODCUTION FROM TRANSESTERIFICATION OF WASTE COOKING OILS USING ALKALINE CATALYSTS M.M. Zamberi 1,2 a, F.N.Ani 1,b and S. N. H. Hassan 2,c 1 Department of Thermodynamics and Fluid
OPTIMIZATION OF BIODIESEL PRODCUTION FROM TRANSESTERIFICATION OF WASTE COOKING OILS USING ALKALINE CATALYSTS M.M. Zamberi 1,2 a, F.N.Ani 1,b and S. N. H. Hassan 2,c 1 Department of Thermodynamics and Fluid
The Purification Feasibilityof GlycerinProduced During
 The Purification Feasibilityof GlycerinProduced During BiodieselProduction S. Soulayman, F. Mustafa, and A. Hadbah Higher Institute for Applied Sciences and technology, Damascus, P.O. Box 31983, Syria,
The Purification Feasibilityof GlycerinProduced During BiodieselProduction S. Soulayman, F. Mustafa, and A. Hadbah Higher Institute for Applied Sciences and technology, Damascus, P.O. Box 31983, Syria,
Biodiesel. As fossil fuels become increasingly expensive to extract and produce, bio-diesel is
 Aaron Paternoster CHEM 380 10D Prof. Laurie Grove January 30, 2015 Biodiesel Introduction As fossil fuels become increasingly expensive to extract and produce, bio-diesel is proving to be an economically
Aaron Paternoster CHEM 380 10D Prof. Laurie Grove January 30, 2015 Biodiesel Introduction As fossil fuels become increasingly expensive to extract and produce, bio-diesel is proving to be an economically
V.Venkatakranthi Teja. N S Raju Institute of Technology (NSRIT), Sontyam, Visakhapatnam, Andhra Pradesh , India.
 Preparation of Waste Cooking Oil as Alternative Fuel and Experimental Investigation Using Bio-Diesel Setup a Comparative Study with Single Cylinder Diesel Engine Mr.S.Sanyasi Rao Pradesh - 531173, India.
Preparation of Waste Cooking Oil as Alternative Fuel and Experimental Investigation Using Bio-Diesel Setup a Comparative Study with Single Cylinder Diesel Engine Mr.S.Sanyasi Rao Pradesh - 531173, India.
Using Response Surface Methodology in Optimisation of Biodiesel Production via Alkali Catalysed Transesterification of Waste Cooking Oil
 Journal of Scientific & Industrial Research Vol. 75, March 2016, pp. 188-193 Using Response Surface Methodology in Optimisation of Biodiesel Production via Alkali Catalysed Transesterification of Waste
Journal of Scientific & Industrial Research Vol. 75, March 2016, pp. 188-193 Using Response Surface Methodology in Optimisation of Biodiesel Production via Alkali Catalysed Transesterification of Waste
Research Article. Synthesis of biodiesel from waste cooking oil by two steps process transesterification and ozonation
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(9S):17-21 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Synthesis of biodiesel from waste cooking oil by
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(9S):17-21 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Synthesis of biodiesel from waste cooking oil by
Production of Biodiesel from Palm Oil by Extractive Reaction
 CHEMICAL ENGINEERING TRANSACTIONS Volume 21, 2010 Editor J. J. Klemeš, H. L. Lam, P. S. Varbanov Copyright 2010, AIDIC Servizi S.r.l., ISBN 978-88-95608-05-1 ISSN 1974-9791 DOI: 10.3303/CET1021206 1231
CHEMICAL ENGINEERING TRANSACTIONS Volume 21, 2010 Editor J. J. Klemeš, H. L. Lam, P. S. Varbanov Copyright 2010, AIDIC Servizi S.r.l., ISBN 978-88-95608-05-1 ISSN 1974-9791 DOI: 10.3303/CET1021206 1231
4. Synthesis of Biodiesel from Palm Fatty Acid Distillate. Research Article
 4. Synthesis of Biodiesel from Palm Fatty Acid Distillate Research Article Abstract Tarun Kataria Third Year Bachelor of Technology Department of Oils, Oleochemicals & Surfactant Technology Palm fatty
4. Synthesis of Biodiesel from Palm Fatty Acid Distillate Research Article Abstract Tarun Kataria Third Year Bachelor of Technology Department of Oils, Oleochemicals & Surfactant Technology Palm fatty
CHAPTER 4 PRODUCTION OF BIODIESEL
 56 CHAPTER 4 PRODUCTION OF BIODIESEL 4.1 INTRODUCTION Biodiesel has been produced on a large scale in the European Union (EU) since 1992 (European Biodiesel Board 2008) and in the United States of America
56 CHAPTER 4 PRODUCTION OF BIODIESEL 4.1 INTRODUCTION Biodiesel has been produced on a large scale in the European Union (EU) since 1992 (European Biodiesel Board 2008) and in the United States of America
BIODIESEL PRODUCTION IN A BATCH REACTOR 1. THEORY
 BIODIESEL PRODUCTION IN A BATCH REACTOR Date: September-November, 2017. Biodiesel is obtained through transesterification reaction of soybean oil by methanol, using sodium hydroxide as a catalyst. The
BIODIESEL PRODUCTION IN A BATCH REACTOR Date: September-November, 2017. Biodiesel is obtained through transesterification reaction of soybean oil by methanol, using sodium hydroxide as a catalyst. The
Experimental Analysis of Cotton Seed oil Biodiesel in a Compression Ignition Engine
 Volume 6, Issue 3, March 217, ISSN: 2278-7798 Experimental Analysis of Cotton Seed oil Biodiesel in a Compression Ignition Engine Allen Jeffrey.J 1,Kiran Kumar.S 2,Antonynishanthraj.R 3,Arivoli.N 4,Balakrishnan.P
Volume 6, Issue 3, March 217, ISSN: 2278-7798 Experimental Analysis of Cotton Seed oil Biodiesel in a Compression Ignition Engine Allen Jeffrey.J 1,Kiran Kumar.S 2,Antonynishanthraj.R 3,Arivoli.N 4,Balakrishnan.P
CONVERSION OF GLYCEROL TO GREEN METHANOL IN SUPERCRITICAL WATER
 CONVERSION OF GLYCEROL TO GREEN METHANOL IN SUPERCRITICAL WATER Maša Knez Hrnčič, Mojca Škerget, Ljiljana Ilić, Ţeljko Knez*, University of Maribor, Faculty of Chemistry and Chemical Engineering, Laboratory
CONVERSION OF GLYCEROL TO GREEN METHANOL IN SUPERCRITICAL WATER Maša Knez Hrnčič, Mojca Škerget, Ljiljana Ilić, Ţeljko Knez*, University of Maribor, Faculty of Chemistry and Chemical Engineering, Laboratory
Use of Ultrasound for Monitoring Reaction Kinetics of Biodiesel Synthesis: Experimental and Theoretical Studies.
 Use of Ultrasound for Monitoring Reaction Kinetics of Biodiesel Synthesis: Experimental and Theoretical Studies. G Ahmad and R Patel University of Bradford Bradford UK Water and Energy Workshop 15 17 February
Use of Ultrasound for Monitoring Reaction Kinetics of Biodiesel Synthesis: Experimental and Theoretical Studies. G Ahmad and R Patel University of Bradford Bradford UK Water and Energy Workshop 15 17 February
Bioprocess Optimization for Biodiesel Production from Pongamia Pinnata
 2013 2nd International Conference on Environment, Energy and Biotechnology IPCBEE vol.51 (2013) (2013) IACSIT Press, Singapore DOI: 10.7763/IPCBEE. 2013. V51. 23 Bioprocess Optimization for Biodiesel Production
2013 2nd International Conference on Environment, Energy and Biotechnology IPCBEE vol.51 (2013) (2013) IACSIT Press, Singapore DOI: 10.7763/IPCBEE. 2013. V51. 23 Bioprocess Optimization for Biodiesel Production
Comparison of Performance of Castor and Mustard Oil with Diesel in a Single and Twin Cylinder Kirsloskar Diesel Engine
 International Journal of Engineering Research and Technology. ISSN 0974-3154 Volume 6, Number 2 (2013), pp. 237-241 International Research Publication House http://www.irphouse.com Comparison of Performance
International Journal of Engineering Research and Technology. ISSN 0974-3154 Volume 6, Number 2 (2013), pp. 237-241 International Research Publication House http://www.irphouse.com Comparison of Performance
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,500 108,000 1.7 M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,500 108,000 1.7 M Open access books available International authors and editors Downloads Our
Synthesis and Characterization of Fatty Acid Methyl Ester by In-Situ Transesterification in Capparis Deciduas Seed
 Synthesis and Characterization of Fatty Acid Methyl Ester by In-Situ Transesterification in Capparis Deciduas Seed Raghunath D POKHARKAR, Prasad E FUNDE, Shripad S JOSHI Shirish S PINGALE Jain irrigation
Synthesis and Characterization of Fatty Acid Methyl Ester by In-Situ Transesterification in Capparis Deciduas Seed Raghunath D POKHARKAR, Prasad E FUNDE, Shripad S JOSHI Shirish S PINGALE Jain irrigation
Determination of phase diagram of reaction system of biodiesel
 324 FEED AND INDUSTRIAL RAW MATERIAL: Industrial Materials and Biofuel Determination of phase diagram of reaction system of biodiesel LIU Ye, YANG Hao, SHE Zhuhua, LIU Dachuan Wuhan Polytechnic University,
324 FEED AND INDUSTRIAL RAW MATERIAL: Industrial Materials and Biofuel Determination of phase diagram of reaction system of biodiesel LIU Ye, YANG Hao, SHE Zhuhua, LIU Dachuan Wuhan Polytechnic University,
Application of Response Surface Methodology in the Statistical Analysis of Biodiesel Production from Microalgae Oil
 Application of Response Surface Methodology in the Statistical Analysis of Biodiesel Production from Microalgae Oil Ikechukwu Fabian Ejim Chemical Engineering Department, Institute of Management and Technology,
Application of Response Surface Methodology in the Statistical Analysis of Biodiesel Production from Microalgae Oil Ikechukwu Fabian Ejim Chemical Engineering Department, Institute of Management and Technology,
Detection of Sulfur Compounds in Natural Gas According to ASTM D5504 with an Agilent Dual Plasma Sulfur Chemiluminescence Detector
 Detection of Sulfur Compounds in Natural Gas According to ASTM D554 with an Agilent Dual Plasma Sulfur Chemiluminescence Detector Application Note Author Rebecca Veeneman Abstract Sulfur compounds in natural
Detection of Sulfur Compounds in Natural Gas According to ASTM D554 with an Agilent Dual Plasma Sulfur Chemiluminescence Detector Application Note Author Rebecca Veeneman Abstract Sulfur compounds in natural
Simultaneous Determination of Fatty Acid Methyl Esters Contents in the Biodiesel by HPLC-DAD Method
 2016 International Conference on Applied Mechanics, Mechanical and Materials Engineering (AMMME 2016) ISBN: 978-1-60595-409-7 Simultaneous Determination of Fatty Acid Methyl Esters Contents in the Biodiesel
2016 International Conference on Applied Mechanics, Mechanical and Materials Engineering (AMMME 2016) ISBN: 978-1-60595-409-7 Simultaneous Determination of Fatty Acid Methyl Esters Contents in the Biodiesel
Kinetics in Hydrolysis of Oils/Fats and Subsequent Methyl Esterification in Two-step Supercritical Methanol Method for Biodiesel Production
 Kinetics in Hydrolysis of ils/fats and Subsequent Methyl Esterification in Two-step Supercritical Methanol Method for Biodiesel Production Eiji Minami and Shiro Saka * Graduate School of Energy Science,
Kinetics in Hydrolysis of ils/fats and Subsequent Methyl Esterification in Two-step Supercritical Methanol Method for Biodiesel Production Eiji Minami and Shiro Saka * Graduate School of Energy Science,
Investigation of Single Cylinder Diesel Engine Using Bio Diesel from Marine Algae
 Investigation of Single Cylinder Diesel Engine Using Bio Diesel from Marine Algae R.Velappan 1, and S.Sivaprakasam 2 1 Assistant Professor, Department of Mechanical Engineering, Annamalai University. Annamalai
Investigation of Single Cylinder Diesel Engine Using Bio Diesel from Marine Algae R.Velappan 1, and S.Sivaprakasam 2 1 Assistant Professor, Department of Mechanical Engineering, Annamalai University. Annamalai
Analysis of Glycerin and Glycerides in Biodiesel (B100) Using ASTM D6584 and EN Application. Author. Abstract. Introduction
 Analysis of Glycerin and Glycerides in Biodiesel (B1) Using ASTM D68 and EN11 Application HPI/Petrochemicals/Polymers Author James D. McCurry Agilent Technologies, Inc. 8 Centerville Road Wilmington, DE
Analysis of Glycerin and Glycerides in Biodiesel (B1) Using ASTM D68 and EN11 Application HPI/Petrochemicals/Polymers Author James D. McCurry Agilent Technologies, Inc. 8 Centerville Road Wilmington, DE
Conventional Homogeneous Catalytic Process with Continuous-typed Microwave and Mechanical Stirrer for Biodiesel Production from Palm Stearin
 2012 4th International Conference on Chemical, Biological and Environmental Engineering IPCBEE vol.43 (2012) (2012) IACSIT Press, Singapore DOI: 10.7763/IPCBEE. 2012. V43. 2 Conventional Homogeneous Catalytic
2012 4th International Conference on Chemical, Biological and Environmental Engineering IPCBEE vol.43 (2012) (2012) IACSIT Press, Singapore DOI: 10.7763/IPCBEE. 2012. V43. 2 Conventional Homogeneous Catalytic
Quantitative Analysis of Chemical Compositions from Various Sources of Crude Glycerine
 CMU.J.Nat.Sci.Special Issue on Agricultural & Natural Resources (2012) Vol.11 (1) 157 Quantitative Analysis of Chemical Compositions from Various Sources of Crude Glycerine Adisorn Settapong * and Chaiyawan
CMU.J.Nat.Sci.Special Issue on Agricultural & Natural Resources (2012) Vol.11 (1) 157 Quantitative Analysis of Chemical Compositions from Various Sources of Crude Glycerine Adisorn Settapong * and Chaiyawan
Gas Chromatographic Analysis of Diesel Fuel Dilution for In-Service Motor Oil Using ASTM Method D7593
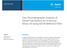 Application Note Gas Chromatographic Analysis of Diesel Fuel Dilution for In-Service Motor Oil Using ASTM Method D7593 Authors Kelly Beard and James McCurry Agilent Technologies, Inc. Abstract An Agilent
Application Note Gas Chromatographic Analysis of Diesel Fuel Dilution for In-Service Motor Oil Using ASTM Method D7593 Authors Kelly Beard and James McCurry Agilent Technologies, Inc. Abstract An Agilent
Biodiesel production by esterification of palm fatty acid distillate
 ARTICLE IN PRESS Biomass and Bioenergy ] (]]]]) ]]] ]]] www.elsevier.com/locate/biombioe Biodiesel production by esterification of palm fatty acid distillate S. Chongkhong, C. Tongurai, P. Chetpattananondh,
ARTICLE IN PRESS Biomass and Bioenergy ] (]]]]) ]]] ]]] www.elsevier.com/locate/biombioe Biodiesel production by esterification of palm fatty acid distillate S. Chongkhong, C. Tongurai, P. Chetpattananondh,
Application of the factorial design of experiments and response surface methodology to optimize biodiesel production
 Industrial Crops and Products 8 (1998) 29 35 Application of the factorial design of experiments and response surface methodology to optimize biodiesel production G. Vicente, A. Coteron, M. Martinez, J.
Industrial Crops and Products 8 (1998) 29 35 Application of the factorial design of experiments and response surface methodology to optimize biodiesel production G. Vicente, A. Coteron, M. Martinez, J.
Towards a Biodiesel-based Biorefinery: Chemical and Physical Properties of Reactively Extracted Rapeseed (Canola)
 Towards a Biodiesel-based Biorefinery: Chemical and Physical Properties of Reactively Extracted Rapeseed (Canola) Yilong Ren, Adam Harvey and Rabitah Zakaria School of Chemical Engineering and Advanced
Towards a Biodiesel-based Biorefinery: Chemical and Physical Properties of Reactively Extracted Rapeseed (Canola) Yilong Ren, Adam Harvey and Rabitah Zakaria School of Chemical Engineering and Advanced
4001 Transesterification of castor oil to ricinoleic acid methyl ester
 4001 Transesterification of castor oil to ricinoleic acid methyl ester castor oil + MeH Na-methylate H Me CH 4 (32.0) C 19 H 36 3 (312.5) Classification Reaction types and substance classes reaction of
4001 Transesterification of castor oil to ricinoleic acid methyl ester castor oil + MeH Na-methylate H Me CH 4 (32.0) C 19 H 36 3 (312.5) Classification Reaction types and substance classes reaction of
Biodiesel Production from Used Cooking Oil using Calcined Sodium Silicate Catalyst
 Biodiesel Production from Used Cooking Oil using Calcined Sodium Silicate Catalyst M.O. Daramola, D. Nkazi, K. Mtshali School of Chemical and Metallurgical Engineering, Faculty of Engineering and the Built
Biodiesel Production from Used Cooking Oil using Calcined Sodium Silicate Catalyst M.O. Daramola, D. Nkazi, K. Mtshali School of Chemical and Metallurgical Engineering, Faculty of Engineering and the Built
Optimization of the Temperature and Reaction Duration of One Step Transesterification
 Optimization of the Temperature and Reaction Duration of One Step Transesterification Ding.Z 1 and Das.P 2 Department of Environmental Science and Engineering, School of Engineering, National university
Optimization of the Temperature and Reaction Duration of One Step Transesterification Ding.Z 1 and Das.P 2 Department of Environmental Science and Engineering, School of Engineering, National university
Abstract Process Economics Program Report 251 BIODIESEL PRODUCTION (November 2004)
 Abstract Process Economics Program Report 251 BIODIESEL PRODUCTION (November 2004) Biodiesel is an ester of fatty acids produced from renewable resources such as virgin vegetable oil, animal fats and used
Abstract Process Economics Program Report 251 BIODIESEL PRODUCTION (November 2004) Biodiesel is an ester of fatty acids produced from renewable resources such as virgin vegetable oil, animal fats and used
PERFORMANCE AND EMISSION CHARACTERISTICS OF DIESEL ENGINE USING RICE BRAN OIL METHYL ESTER BLEND WITH ADITIVE DIETHYL ETHER (DEE)
 International Journal of Science, Engineering and Technology Research (IJSETR), Volume 3, Issue 2, February 214 PERFORMANCE AND EMISSION CHARACTERISTICS OF DIESEL ENGINE USING RICE BRAN OIL METHYL ESTER
International Journal of Science, Engineering and Technology Research (IJSETR), Volume 3, Issue 2, February 214 PERFORMANCE AND EMISSION CHARACTERISTICS OF DIESEL ENGINE USING RICE BRAN OIL METHYL ESTER
Treatment of BDF Wastewater with Hydrothermal Electrolysis
 Treatment of BDF Wastewater with Hydrothermal Electrolysis Asli YUKSEL 1, Hiromichi KOGA 1, Mitsuru SASAKI 1 * and Motonobu GOTO 2 1 Graduate School of Science and Technology, Kumamoto University, JAPAN
Treatment of BDF Wastewater with Hydrothermal Electrolysis Asli YUKSEL 1, Hiromichi KOGA 1, Mitsuru SASAKI 1 * and Motonobu GOTO 2 1 Graduate School of Science and Technology, Kumamoto University, JAPAN
OPTIMIZATION OF IN-SITU TRANSESTERIFICATION PROCESS OF BIODIESEL FROM NYAMPLUNG (Calophyllum inophyllum L.) SEED USING MICROWAVE
 Rasayan J. Chem., 10(3), 952-958(2017) http://dx.doi.org/10.7324/rjc.2017.1031803 Vol. 10 No. 3 952-958 July - September 2017 ISSN: 0974-1496 e-issn: 0976-0083 CODEN: RJCABP http://www.rasayanjournal.com
Rasayan J. Chem., 10(3), 952-958(2017) http://dx.doi.org/10.7324/rjc.2017.1031803 Vol. 10 No. 3 952-958 July - September 2017 ISSN: 0974-1496 e-issn: 0976-0083 CODEN: RJCABP http://www.rasayanjournal.com
A Novel Non-catalytic Biodiesel Production Process by Supercritical Methanol as NEDO High Efficiency Bioenergy Conversion Project
 A Novel Non-catalytic Biodiesel Production Process by Supercritical Methanol as NEDO High Efficiency Bioenergy Conversion Project Shiro Saka * and Eiji Minami Graduate School of Energy Science, Kyoto University,
A Novel Non-catalytic Biodiesel Production Process by Supercritical Methanol as NEDO High Efficiency Bioenergy Conversion Project Shiro Saka * and Eiji Minami Graduate School of Energy Science, Kyoto University,
Methanol in Biodiesel by EN14110 with the HT3 and Versa Automated Headspace Analyzers. Versa HT3. Application Note. Abstract.
 Methanol in Biodiesel by EN14110 with the HT3 and Versa Automated Headspace Analyzers Application Note Abstract Versa With the rising prices of fossil fuels, more emphasis is being put on renewable resources
Methanol in Biodiesel by EN14110 with the HT3 and Versa Automated Headspace Analyzers Application Note Abstract Versa With the rising prices of fossil fuels, more emphasis is being put on renewable resources
ScienceDirect. Biodiesel production in supercritical methanol using a novel spiral reactor
 Available online at www.sciencedirect.com ScienceDirect Procedia Environmental Sciences 28 (215 ) 24 213 The 5th Sustainable Future for Human Security (SustaiN 214) Biodiesel production in supercritical
Available online at www.sciencedirect.com ScienceDirect Procedia Environmental Sciences 28 (215 ) 24 213 The 5th Sustainable Future for Human Security (SustaiN 214) Biodiesel production in supercritical
International Journal of ChemTech Research CODEN (USA): IJCRGG ISSN: Vol.7, No.4, pp ,
 International Journal of ChemTech Research CODEN (USA): IJCRGG ISSN: 0974-4290 Vol.7, No.4, pp 2112-2116, 2014-2015 Production of Biodiesel by Transesterification of Algae Oil with an assistance of Nano-CaO
International Journal of ChemTech Research CODEN (USA): IJCRGG ISSN: 0974-4290 Vol.7, No.4, pp 2112-2116, 2014-2015 Production of Biodiesel by Transesterification of Algae Oil with an assistance of Nano-CaO
Transesterification of Palm Oil to Biodiesel and Optimization of Production Conditions i.e. Methanol, Sodium Hydroxide and Temperature
 Journal of Energy and Natural Resources 2015; 4(3): 45-51 Published online June 18, 2015 (http://www.sciencepublishinggroup.com/j/jenr) doi: 10.11648/j.jenr.20150403.12 ISSN: 2330-7366 (Print); ISSN: 2330-7404
Journal of Energy and Natural Resources 2015; 4(3): 45-51 Published online June 18, 2015 (http://www.sciencepublishinggroup.com/j/jenr) doi: 10.11648/j.jenr.20150403.12 ISSN: 2330-7366 (Print); ISSN: 2330-7404
Biodiesel Solutions André Y. Tremblay, P.Eng., Ph.D. Department of Chemical and Biological Engineering University of Ottawa
 Biodiesel Solutions André Y. Tremblay, P.Eng., Ph.D. Department of Chemical and Biological Engineering University of Ottawa PEO - Ottawa Chapter- Sustainability Seminar January 24 th, 2013 CO2 and Temperature
Biodiesel Solutions André Y. Tremblay, P.Eng., Ph.D. Department of Chemical and Biological Engineering University of Ottawa PEO - Ottawa Chapter- Sustainability Seminar January 24 th, 2013 CO2 and Temperature
Analysis of Mahua Biodiesel Production with Combined Effects of Input Trans-Esterification Process Parameters
 INTERNATIONAL JOURNAL OF R&D IN ENGINEERING, SCIENCE AND MANAGEMENT Vol.3, Issue 7, April 2016, p.p.297-301, ISSN 2393-865X Analysis of Mahua Biodiesel Production with Combined Effects of Input Trans-Esterification
INTERNATIONAL JOURNAL OF R&D IN ENGINEERING, SCIENCE AND MANAGEMENT Vol.3, Issue 7, April 2016, p.p.297-301, ISSN 2393-865X Analysis of Mahua Biodiesel Production with Combined Effects of Input Trans-Esterification
Saddam H. Al-lwayzy. Supervisors: Dr. Talal Yusaf Dr. Paul Baker Dr. Troy Jensen 3/24/2013 1
 Saddam H. Al-lwayzy Supervisors: Dr. Talal Yusaf Dr. Paul Baker Dr. Troy Jensen 3/24/2013 1 1. Introduction 2. Literature review 3. Research aim 4. Methodology 5. Some results 3/24/2013 2 Introduction
Saddam H. Al-lwayzy Supervisors: Dr. Talal Yusaf Dr. Paul Baker Dr. Troy Jensen 3/24/2013 1 1. Introduction 2. Literature review 3. Research aim 4. Methodology 5. Some results 3/24/2013 2 Introduction
Determination of Free and Total Glycerin in B100 Biodiesel
 Page 1 of 5 Page 1 of 5 Return to Web Version Determination of Free and Total Glycerin in B100 Biodiesel By: Michael D. Buchanan, Katherine K. Stenerson, and Vicki Yearick, Reporter US Vol 27.1 techservice@sial.com
Page 1 of 5 Page 1 of 5 Return to Web Version Determination of Free and Total Glycerin in B100 Biodiesel By: Michael D. Buchanan, Katherine K. Stenerson, and Vicki Yearick, Reporter US Vol 27.1 techservice@sial.com
Improving the Quality and Production of Biogas from Swine Manure and Jatropha (Jatropha curcas) Seeds
 Improving the Quality and Production of Biogas from Swine Manure and Jatropha (Jatropha curcas) Seeds Amy Lizbeth J. Rico Company: Tarlac Agricultural University College of Engineering Technology Address:
Improving the Quality and Production of Biogas from Swine Manure and Jatropha (Jatropha curcas) Seeds Amy Lizbeth J. Rico Company: Tarlac Agricultural University College of Engineering Technology Address:
A Novel Membrane Reactor for Production of High-Purity Biodiesel
 European Online Journal of Natural and Social Sciences 2014; www.european-science.com Vol.3, No.3 Special Issue on Environmental, Agricultural, and Energy Science ISSN 1805-3602 A Novel Membrane Reactor
European Online Journal of Natural and Social Sciences 2014; www.european-science.com Vol.3, No.3 Special Issue on Environmental, Agricultural, and Energy Science ISSN 1805-3602 A Novel Membrane Reactor
Increased sensitivity and reproducibility in the analysis of trace fatty acid methyl esters in jet fuel
 Application Note Energy and Chemicals Increased sensitivity and reproducibility in the analysis of trace fatty acid methyl esters in jet fuel Applying the Energy Institute Method IP 8 with an Agilent J&W
Application Note Energy and Chemicals Increased sensitivity and reproducibility in the analysis of trace fatty acid methyl esters in jet fuel Applying the Energy Institute Method IP 8 with an Agilent J&W
Application Of Response Surface Methodology In The Optimization Of Biodiesel Production From Microalgae Oil
 Journal of Multidisciplinary Engineering Science and Technology (JMEST) Application Of Response Surface Methodology In The Optimization Of Biodiesel Production From Microalgae Oil * Kamen, F.L; Ejim, I.F;
Journal of Multidisciplinary Engineering Science and Technology (JMEST) Application Of Response Surface Methodology In The Optimization Of Biodiesel Production From Microalgae Oil * Kamen, F.L; Ejim, I.F;
Proposal to Determine Various Properties of Biodiesel Fuels Based on Methyl Ester. Composition. Jason Freischlag. Dr. Porter Chem /25/2013
 1 Proposal to Determine Various Properties of Biodiesel Fuels Based on Methyl Ester Composition Jason Freischlag Dr. Porter Chem 402 11/25/2013 2 Specific Aims Biodiesel is an alternative fuel source that
1 Proposal to Determine Various Properties of Biodiesel Fuels Based on Methyl Ester Composition Jason Freischlag Dr. Porter Chem 402 11/25/2013 2 Specific Aims Biodiesel is an alternative fuel source that
PROJECT REFERENCE NO.: 39S_R_MTECH_1508
 DEVELOPMENT OF AGRICULTURAL WASTE BASED HETEROGENEOUS CATALYST FOR PRODUCTION OF BIODIESEL FROM MIXED WASTE COOKING OIL AND ITS PERFORMANCE ON DIESEL ENGINE PROJECT REFERENCE NO.: 39S_R_MTECH_1508 COLLEGE
DEVELOPMENT OF AGRICULTURAL WASTE BASED HETEROGENEOUS CATALYST FOR PRODUCTION OF BIODIESEL FROM MIXED WASTE COOKING OIL AND ITS PERFORMANCE ON DIESEL ENGINE PROJECT REFERENCE NO.: 39S_R_MTECH_1508 COLLEGE
Lipase-Catalyzed Biodiesel Production with Methyl Acetate as Acyl Acceptor
 Lipase-Catalyzed Biodiesel Production with Methyl Acetate as Acyl Acceptor Ying Huang and Yunjun Yan* School of Life Science & Technology, Huazhong University of Science & Technology, Wuhan 430074, P.
Lipase-Catalyzed Biodiesel Production with Methyl Acetate as Acyl Acceptor Ying Huang and Yunjun Yan* School of Life Science & Technology, Huazhong University of Science & Technology, Wuhan 430074, P.
Process units needed to make biodiesel continuously. Michael Allen Department of Mechanical Engineering Prince of Songkla University Thailand
 Process units needed to make biodiesel continuously Michael Allen Department of Mechanical Engineering Prince of Songkla University Thailand Why continuous? #For a reactor having volume V R and mean residence
Process units needed to make biodiesel continuously Michael Allen Department of Mechanical Engineering Prince of Songkla University Thailand Why continuous? #For a reactor having volume V R and mean residence
What is Biodiesel? Biodiesel consists of alkyl-esters derived from a biological source
 Biodiesel What is Biodiesel? Biodiesel consists of alkyl-esters derived from a biological source Biodiesel can be used as a fuel in compression ignition engines (i.e. diesels) Can be blended with petroleum
Biodiesel What is Biodiesel? Biodiesel consists of alkyl-esters derived from a biological source Biodiesel can be used as a fuel in compression ignition engines (i.e. diesels) Can be blended with petroleum
Agilent 7696A Sample Prep WorkBench Automated Sample Preparation for the GC Analysis of Biodiesel Using Method EN14105:2011
 Agilent 7696A Sample Prep WorkBench Automated Sample Preparation for the GC Analysis of Biodiesel Using Method EN14105:2011 Application Note Fuels Author James D. McCurry, Ph.D. Agilent Technologies, Inc.
Agilent 7696A Sample Prep WorkBench Automated Sample Preparation for the GC Analysis of Biodiesel Using Method EN14105:2011 Application Note Fuels Author James D. McCurry, Ph.D. Agilent Technologies, Inc.
RESEARCH REPORT PRODUCTION OF BIODIESEL FROM CHICKEN FAT WITH COMBINATION SUBCRITICAL METHANOL AND WATER PROCESS
 RESEARCH REPORT PRODUCTION OF BIODIESEL FROM CHICKEN FAT WITH COMBINATION SUBCRITICAL METHANOL AND WATER PROCESS Submitted by: Felix Harijaya Santosa NRP. 5203014015 Ryan Sumule NRP. 5203014037 DEPARTMENT
RESEARCH REPORT PRODUCTION OF BIODIESEL FROM CHICKEN FAT WITH COMBINATION SUBCRITICAL METHANOL AND WATER PROCESS Submitted by: Felix Harijaya Santosa NRP. 5203014015 Ryan Sumule NRP. 5203014037 DEPARTMENT
Material Science Research India Vol. 7(1), (2010)
 Material Science Research India Vol. 7(1), 201-207 (2010) Influence of injection timing on the performance, emissions, combustion analysis and sound characteristics of Nerium biodiesel operated single
Material Science Research India Vol. 7(1), 201-207 (2010) Influence of injection timing on the performance, emissions, combustion analysis and sound characteristics of Nerium biodiesel operated single
Utilization of Three Non-Edible Vegetable Oils for the Production of Biodiesel Catalysed by Enzyme
 The Open Chemical Engineering Journal, 2008, 2, 79-83 79 Open Access Utilization of Three Non-Edible Vegetable Oils for the Production of Biodiesel Catalysed by Enzyme Sandip Kumar Haldar and Ahindra Nag*
The Open Chemical Engineering Journal, 2008, 2, 79-83 79 Open Access Utilization of Three Non-Edible Vegetable Oils for the Production of Biodiesel Catalysed by Enzyme Sandip Kumar Haldar and Ahindra Nag*
High Temperature Simulated Distillation Performance Using the Agilent 8890 Gas Chromatograph
 Application Note Petrochemicas High Temperature Simulated Distillation Performance Using the Agilent 8890 Gas Chromatograph Author James D. McCurry, Ph.D. Agilent Technologies, Inc. Abstract An Agilent
Application Note Petrochemicas High Temperature Simulated Distillation Performance Using the Agilent 8890 Gas Chromatograph Author James D. McCurry, Ph.D. Agilent Technologies, Inc. Abstract An Agilent
Optimization of Esterification and Transesterification of High FFA Jatropha Curcas Oil Using Response Surface Methodology
 Optimization of Esterification and Transesterification of High FFA Jatropha Curcas Oil Using Surface Methodology Prerna Goyal *1, M.P. Sharma 2, Siddharth Jain 3 Biofuel Research Laboratory, Alternate
Optimization of Esterification and Transesterification of High FFA Jatropha Curcas Oil Using Surface Methodology Prerna Goyal *1, M.P. Sharma 2, Siddharth Jain 3 Biofuel Research Laboratory, Alternate
Direct Production of Biodiesel from Lipid-Bearing Materials, Including Canola
 Direct Production of Biodiesel from Lipid-Bearing Materials, Including Canola 1 Abstract Michael J. Haas, Karen Scott, Thomas Foglia and William N. Marmer Eastern Regional Research Center Agricultural
Direct Production of Biodiesel from Lipid-Bearing Materials, Including Canola 1 Abstract Michael J. Haas, Karen Scott, Thomas Foglia and William N. Marmer Eastern Regional Research Center Agricultural
COMBUSTION CHARACTERISTICS OF DI-CI ENGINE WITH BIODIESEL PRODUCED FROM WASTE CHICKEN FAT
 COMBUSTION CHARACTERISTICS OF DI-CI ENGINE WITH BIODIESEL PRODUCED FROM WASTE CHICKEN FAT K. Srinivasa Rao Department of Mechanical Engineering, Sai Spurthi Institute of Technology, Sathupally, India E-Mail:
COMBUSTION CHARACTERISTICS OF DI-CI ENGINE WITH BIODIESEL PRODUCED FROM WASTE CHICKEN FAT K. Srinivasa Rao Department of Mechanical Engineering, Sai Spurthi Institute of Technology, Sathupally, India E-Mail:
address: (K. A. Younis), (J. L. Ismail Agha), (K. S.
 American Journal of Applied Chemistry 2014; 2(6): 105-111 Published online November 28, 2014 (http://www.sciencepublishinggroup.com/j/ajac) doi: 10.11648/j.ajac.20140206.12 ISSN: 2330-8753 (Print); ISSN:
American Journal of Applied Chemistry 2014; 2(6): 105-111 Published online November 28, 2014 (http://www.sciencepublishinggroup.com/j/ajac) doi: 10.11648/j.ajac.20140206.12 ISSN: 2330-8753 (Print); ISSN:
