Poon, Hiew Mun; Pang, Kar Mun; Ng, Hoon Kiat; Gan, Suyin; Schramm, Jesper
|
|
|
- Bryce Snow
- 5 years ago
- Views:
Transcription
1 Downloaded from orbit.dtu.dk on: Nov 09, 2018 Development of multi-component diesel surrogate fuel models Part I: Validation of reduced mechanisms of diesel fuel constituents in 0-D kinetic simulations Poon, Hiew Mun; Pang, Kar Mun; Ng, Hoon Kiat; Gan, Suyin; Schramm, Jesper Published in: Fuel Link to article, DOI: /j.fuel Publication date: 2016 Document Version Peer reviewed version Link back to DTU Orbit Citation (APA): Poon, H. M., Pang, K. M., Ng, H. K., Gan, S., & Schramm, J. (2016). Development of multi-component diesel surrogate fuel models Part I: Validation of reduced mechanisms of diesel fuel constituents in 0-D kinetic simulations. Fuel, 180, DOI: /j.fuel General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim.
2 Development of Multi-Component Diesel Surrogate Fuel Models - Part I: Validation of Reduced Mechanisms of Diesel Fuel Constituents in 0-D Kinetic Simulations Hiew Mun Poon 1, Kar Mun Pang 2, Hoon Kiat Ng 1*, Suyin Gan 3, Jesper Schramm 2 1 Department of Mechanical, Materials and Manufacturing Engineering, The University of Nottingham Malaysia Campus, Jalan Broga, Semenyih, Selangor, Malaysia. 2 Department of Mechanical Engineering, Danmarks Tekniske Universitet, Nils Koppels Allé, Bygning 403, 2800 Kgs. Lyngby. 3 Department of Chemical and Environmental Engineering, The University of Nottingham Malaysia Campus, Jalan Broga, Semenyih, Selangor, Malaysia. * Corresponding author: Tel: ; Fax: ; -address: hoonkiat.ng@nottingham.edu.my (H.K. Ng) Abstract In the present work, development and validation of reduced chemical kinetic mechanisms for several different hydrocarbons are performed. These hydrocarbons are potential representative for practical diesel fuel constituents. n-hexadecane (HXN), 2,2,4,4,6,8,8- heptamethylnonane (HMN), cyclohexane (CHX) and toluene are selected to represent straight-alkane, branched-alkane, cyclo-alkane and aromatic compounds in the diesel fuel. A five-stage chemical kinetic mechanism reduction scheme formulated in the previous work is applied to develop the reduced HMN and CHX models based on their respective detailed mechanisms. Alongside with the development of the reduced CHX model, a skeletal toluene sub-mechanism is constructed since the elementary reactions for toluene are subset of the detailed CHX mechanism. The final reduced HMN mechanism comprises 89 species with 319 elementary reactions, while the final reduced CHX mechanism which includes the toluene sub-mechanism consists of 80 species with 287 elementary reactions. Both reduced models are approximately 92 % smaller than their respective detailed models in terms of total 1
3 number of species and elementary reactions. Following that, both the newly developed fuel constituent reduced mechanisms, together with the formerly derived reduced HXN mechanism are comprehensively validated in zero-dimensional chemical kinetic simulations under a wide range of shock tube and jet-stirred reactor (JSR) conditions. Well agreement between the reduced and detailed mechanisms is achieved for ignition delay (ID) and species concentration predictions under both auto-ignition and JSR conditions, with a maximum relative error of 40 %. In addition, the reduced models are further validated against the JSR experimental results for each diesel fuel constituents. The surrogate models are able to reasonably reproduce the experimental species concentration profiles in view of their simplified fuel chemistries. Deviations within one order of magnitude in the absolute values are recorded between the computations and measurements. Validation of these reduced models for each diesel fuel constituents in this work serves as a prerequisite for constructing a multi-component diesel surrogate fuel model. The compact yet accurate chemical models proposed here aid to reduce the chemistry size of the final multi-component diesel surrogate model such that it remains computationally efficient when it is incorporated with multidimensional CFD simulations. The reduced mechanism of each fuel constituent can also be used individually for other CFD applications. Keywords: mechanism reduction, diesel fuel, branched-alkanes, cyclo-alkanes, aromatics Introduction Diesel fuels primarily comprise complex mixtures of different types of hydrocarbon species that can be categorised into several basic structural classes of compounds such as straightalkanes (also known as n-alkanes), branched-alkanes (also known as iso-alkanes), cycloalkanes, and aromatic compounds. For example, typical North American diesel fuels generally consist of 25 % to 50 % of straight- and branched-alkanes, 20 % to 40 % of cycloalkanes as well as 15 % to 40 % of aromatic compounds [1]. 2
4 Among all these classes, straight-alkanes are usually the most abundant components in liquid fuels [2,3]. For the past decades, surrogate models with short carbon chain, such as n- heptane, are generally employed as the representatives for diesel fuels. Nonetheless, it is found that the short-chain surrogate fuel models are inadequate to represent the combustion kinetics of the actual fuels [1,4,5]. Hence, surrogate models for long-chain, straight-alkanes such as n-dodecane, n-tetradecane, and n-hexadecane (HXN) are developed owing to their comparable boiling range to those of the diesel fuels [6]. Hence, these models can potentially be used as diesel fuel surrogate models in multi-dimensional computational fluid dynamics (CFD) modelling studies. In particular, HXN is one of the favourable options as it is the diesel primary reference fuel which permits fuel blending with greater extent of cetane number (CN) range. Of late, there has been considerable progress on the development of chemical kinetic mechanisms for HXN [6 9]. Apart from the kinetic modelling studies, it is noteworthy that HXN has also started to gain popularity in multi-dimensional numerical simulations. For instance, a reduced HXN mechanism with 381 species was successfully derived from the parent mechanism in the study of Liang et al. [10]. The reduced model was subsequently used to simulate homogeneous charge compression ignition (HCCI) combustion of HXN. In addition to that, diesel spray combustion in a constant volume combustion chamber was also simulated by Poon et al. [11] using a compact HXN model. Despite the various reported success of these HXN models, employing HXN alone as a single-component diesel fuel surrogate is not suggested owing to its different hydrogen/carbon molar ratio (H/C) as compared to that of the actual diesel fuels [5,12]. Hence, it is recommended to integrate HXN with other diesel fuel constituents in a surrogate blend to match the diesel fuel kinetics, compositions and CN The iso-alkanes are usually lightly branched with only one or two methyl substituents on a long carbon chain [2,3]. To date, iso-octane is the most studied branched-alkane and it has an 3
5 octane number of 100 [13]. It is a primary reference fuel for gasoline that is difficult to ignite [14]. On the other hand, the highly branched 2,2,4,4,6,8,8-heptamethylnonane (HMN), which is also known as iso-cetane, has been gaining increasing attention as it is a cetane rating reference compound for diesel fuel with CN of 15. This ignition property is important in producing fuel mixtures with different CN when it is integrated with HXN. In view of its importance as a potential surrogate component for the diesel fuels, recent studies have been carried out using HMN with the use of experimental and kinetic modelling approaches [15 18]. A novel chemical kinetic model has been generated by Oehlschlaeger et al. [18] to describe the oxidation of HMN based on the formerly derived iso-octane mechanism [19]. The model has successfully captured the ignition delay (ID) behaviour of HMN oxidation under shock-tube conditions, which is also the first ID measurement for HMN thus far. Although multi-dimensional CFD modelling for HMN fuel combustion has yet to be reported in the literature, a reduced model is expected to be useful for the integration with HXN mechanism to produce a compact diesel surrogate fuel model On the other hand, cyclo-alkanes which consist of a cyclic structure, are an important chemical class of hydrocarbons found in diesel fuels [20,21]. It is noted that the amount of cyclo-alkanes in the fuels might influence the fuel ignition quality as well as soot emission performances [21]. The presence of cyclo-alkane components in the fuels can increase soot production as they generate aromatic compounds through dehydrogenation process, which acts as the inception sites for soot growth [21]. Based on literature, the simplest forms of cyclo-alkanes, namely cyclopentane and cyclohexane (CHX), have received much attention in both experimental investigations and modelling work lately [20 24]. These two components have been widely studied in shock tubes [22,25 27], jet-stirred reactors (JSR) [20,23,28], laminar pre-mixed flames [24,29] and plug flow reactors [30]. According to the modelling study of Ra and Reitz [31], CHX has been chosen as a surrogate to represent the 4
6 cyclo-alkane component in the diesel fuels. This representative surrogate is successively applied to formulate a multi-component chemical kinetic model for CFD modelling of HCCI and direct injection engine combustion. Apart from that, more recently, a 50-species reduced chemical kinetic mechanism for CHX has also been successfully incorporated into the multidimensional CFD code to model the auto-ignition processes in an rapid compression machine (RCM) under HCCI engine-like conditions [32] Last but not least, aromatics compounds in diesel fuels are cyclic, planar hydrocarbons with alternating double and single bonds between carbon atoms, forming a continuous ring [2]. Based on the review of Farrell et al. [1], toluene is recommended as the near-term surrogate component for aromatics, by taking into the considerations of the availability of kinetic models and data for all aromatic compounds. Besides, toluene is often integrated into various diesel surrogate fuel blends [33 35] for soot formation modelling as it plays an important role in the formation of polycyclic aromatic hydrocarbons (PAH) and soot precursors. Inclusion of aromatics as a diesel fuel constituent is expected to improve the predictions of soot formation process Set against this background, this study aims to develop reduced chemical models which are good representatives for branched-alkanes, cyclo-alkanes and aromatic compounds in typical diesel fuel. The reduced models for each diesel fuel components are then comprehensively validated in zero-dimensional (0-D) chemical kinetic simulations under a wide range of shock tube and JSR conditions. Similar validation exercises are also carried out on the previously derived reduced HXN mechanism which is a surrogate component for straight-alkane in the diesel fuel model Development of Reduced Chemical Kinetic Mechanisms for Diesel Fuel Constituents 5
7 In this work, HXN [6], HMN [18], CHX [21] and toluene [21] are proposed to represent the diesel fuel components. The straight-alkane, HXN, and the branched-alkane, HMN, are diesel primary reference fuels. HMN has a CN of 15 and its combination with HXN provides the capability to vary the CN of the diesel surrogate fuel models. CHX is selected to represent the cycloalkane component in diesel fuel as it is capable of representing the major reaction characteristics of cyclo-alkane oxidation process even though it is the simplest compound [21]. Besides, it may also have a greater effect on soot production as compared to non-cyclic alkane whereby the oxidation routes directly yield aromatic species as intermediates [1]. On the other hand, toluene has one of the simplest molecular structures of the alkylated benzenes and it is regarded as a good representative of the characteristics of aromatic fuels [36] It is noteworthy that all these fuel constituent models are essentially constructed based on the hierarchical nature of hydrocarbon oxygen systems [6,18,21] which take into account the kinetics of H 2 /CO oxidations as well as small hydrocarbon species which are typically formed in fuel decomposition. These pools of species can then be shared by the fuel constituents in the final multi-component diesel surrogate fuel mechanisms during the model integration [37,38] Five-Stage Chemical Kinetic Mechanism Reduction Scheme The five-stage chemical kinetic mechanism reduction scheme developed in the previous work [11,39] is applied to derive reduced mechanisms for each diesel fuel components. The reduction scheme is illustrated in Fig. A1 in Appendix A. The mechanism reduction scheme consists of five reduction phases such as Directed Relation Graph with Error Propagation (DRGEP) [40,41] using Dijkstra s algorithm [42], isomer lumping [43,44], reaction path analysis, Directed Relation Graph (DRG) [45,46] as well as adjustment of reaction rate constant [37,44,47,48]. 6
8 During the DRGEP reduction, CO, CO 2, HCO, HO 2, H 2 O 2, H 2, and N 2 are chosen as target species to determine their shortest pathways to all other species using the Dijkstra s algorithm [42]. A Direct Interaction Coefficient (DIC) is applied to calculate the dependency of a species on another based on its impact to the total production or consumption rate. DIC of species y to the production rate of species x is expressed by the following equation: 153 DIC = N R v x,i ω i δi i=1 y N max( R N max(0,v x,i ω i ), R max(0, v i=1 x,i ω i )) i=1 Px Cx (1) where N R is the total number of elementary reactions in the mechanism. v x,i is the stoichiometric coefficient of species x while ω i is the net production rate of i th elementary reaction. P x is the overall production rate of species x and C x is the overall consumption rate of species x. δ yi denotes the participation of species y in i th elementary reaction and it is defined as: 159 δ yi = { 1, 0, if the i th elementary reaction involves species y; otherwise; (2) Then, an Overall Interaction Coefficient, R xy, is introduced to determine the maximum of all dependency pathways for all species relative to the targets. It is defined as: 162 N S 1 r sj s j+1 R xy = max all path p ( j=1 ) (3) where j refers to the j th species of pathway p. S is the placeholder for the intermediate species which starts at species x and ends at species y. N S is the total number of species in the mechanism. r is equivalent to DIC presented in Equation (1). Here, the error tolerance set for the DRGEP reduction is 5x10-4. Hence, species with R xy values lower than the user-specified tolerance are eliminated from the mechanism. 7
9 Accordingly, species with similar thermodynamic and transport properties are lumped together while insignificant isomers with concentration level lower than 1x10-10 mole/cm 3 are eliminated Following that, main reaction pathways during the fuel oxidation process are analysed using the reaction path analyser of CHEMKIN-PRO software and reactions of species with low normalised temperature A-factor sensitivity are removed. The normalised temperature A- factor sensitivity is expressed by Equation (4) [49]: 175 Normalised Temperature A Factor Sensitivity 176 = Individual Temperature Sensitivity Coefficient Value Total Temperature Sensitivity Coeffcient Value (4) Subsequently, DRG is performed to remove species which have lost pathway connection to the fuel species. Here, the threshold limit is set to unity in order to identify all the coupled species through graph searching using a species coupling measure, r xy. r xy is a normalised contribution of species y to the production rate of species x and it is expressed as: 181 r xy = i=1,n v R x,iω i δ yi (5) i=1,n R v x,i ω i Large-scale elimination of species and reactions from the detailed mechanisms commonly causes deviations in the ID and species concentration predictions. Consequently, appropriate optimisation of the reaction rate constants is carefully performed [37,44,47,48]. Further description of the mechanism reduction scheme is detailed in the previous work [11,39] Fuel Constituent Base Mechanisms for Kinetic Model Reduction Here, detailed models of HXN (2,116 species, 8,130 elementary reactions), HMN (1,114 species, 4,469 elementary reactions) and CHX (1,081 species, 4,269 elementary reactions) developed by Westbrook et al. [6], Oehlschlaeger et al. [18] and Silke et al. [21], respectively 8
10 are employed as parent mechanisms for kinetic model reduction. The reduced model for HXN comprising 79 species with 289 elementary reactions has been derived in the previous work [11] and thus it is directly applied here. Also, the elementary reactions for toluene are subset of the detailed mechanism of CHX. Both CHX and toluene are important components for benzene production, which acts as a connecting species between them through several reaction pathways. Consequently, the mechanism reduction is carried out for the detailed mechanisms of HMN and CHX in the current study as there is no reported work on the formulation of these mechanisms beforehand Mechanism Reduction Procedures Similar to the previous mechanism reduction exercise [11], capability of the mechanisms in auto-ignition predictions is selected as the basis for reduction. Both JSR and auto-ignition conditions are then chosen as data source for mechanism reduction, where sampled data points are obtained over a wide range of initial pressure, initial temperature and equivalence ratio (Ф). The conditions applied for mechanism reduction are demonstrated in Table 1. JSR is included in this study as an additional data source for mechanism reduction as it is important in modelling the steady-state extinction process of the combustion process [47]. Closed homogeneous batch reactor and Perfectly-Stirred Reactor (PSR) models of CHEMKIN-PRO software are applied As a result, a reduced HMN mechanism comprising 89 species with 319 elementary reactions and a reduced CHX mechanism comprising 80 species with 287 elementary reactions are successfully derived from the integrated mechanism reduction scheme. In this reduction work, appropriate optimisation of the reaction rate constants are carried out such that the influence of the eliminated reactions is incorporated in the Arrhenius rate constants of the retained reactions in order to maintain accuracy of the model predictions [33,37,48]. As such, the relative errors in the ID and fuel concentration predictions induced by the reduction 9
11 procedure are minimised as compared to the computations of the detailed models. This reaction rate constant optimisation approach has also been successfully demonstrated in the modelling studies of Wang et al. [37,48] and Golovitchev et al. [33]. The approach had significantly improved their model predictions where the reaction rate constants of H-atom abstraction from the fuel species was included as one of their optimisation targets, as with this study. A perturbation by more than a factor of 30 in the A-factor Arrhenius constant was also reported in the study of Wang et al. [37]. The optimised rate constants for important reactions in these reduced mechanisms and the associated targeted functions are detailed in Table 2. The rate constant tuning is carried out for reactions with high normalised temperature A-factor sensitivity across all the test conditions, as shown in Fig. A2 in Appendix A. The results are calculated based on the temperature sensitivity coefficient values with initial pressure of 60 bar, initial temperature of 950 K and Ф of 1. Results here are demonstrated to provide a clearer description on the rate constant tuning procedure It is observed that not all of the reactions with high temperature sensitivity coefficient values are selected for rate constant tuning. The procedure is performed according to the targeted functions such as improvement of ID at low temperatures and thus changes in ID at other temperature regimes are undesirable. As a result, reactions with high temperature sensitivity coefficient values which alter IDs at other temperature regimes are not taken into consideration in this case. In order to ensure the IDs at other temperature regimes across all the test conditions are not affected, the 0-D simulations are repeated whenever the rate constant is adjusted. The reaction rate optimisation process is performed in the following steps: (i) Sensitivity analysis is carried out for each diesel fuel constituent model by applying the test conditions illustrated in Table 1. Reactions with high normalised temperature A- 10
12 factor sensitivity (> 0.1) are taken into consideration for the subsequent optimisation procedure (ii) The A-factor Arrhenius constants for reactions that have significant effects on the ID predictions are calibrated. Concurrently, the resulting key species profiles computed by the reduced models are also monitored. Here, optimisation of the reaction rate constants is performed in order to match the results of the detailed models throughout all the shock tube conditions (iii) Calibration of the A-factor Arrhenius constants is subsequently carried out to reproduce the key species concentrations under JSR conditions. For instance, results obtained from the sensitivity analysis reveal that reactions (R1)-(R2), (R6) and (R11) shown in Table 2 have significant effects on the consumptions of fuel species such as HXN, HMN and C 6 H 5 CH 3 during oxidation process. Hence, the associated A-factor values are adjusted to match the fuel concentration predictions of the detailed models (iv) Steps (ii) and (iii) are repeated until satisfactory results in ID and species profiles predictions throughout all the test conditions are obtained. The induced error for each prediction is retained to an error tolerance of 40 %, which is reasonable for large-scale mechanism reduction as reported in the literature [44,51 53]. It should be noted that all the calibrations are first performed by modifying the order of magnitude of the A-factor value in order to examine its impact on the model predictions. Further optimisation is then carried out by just altering the values within the same order of magnitude to achieve higher accuracy The major reaction pathways for each diesel fuel component are illustrated in Fig. A3 in Appendix A. The key reaction pathways of the fuel combustion are obtained from reaction pathway analysis during the mechanism reduction process. As observed in the fuel oxidation 11
13 pathways under similar conditions, the oxidation of n-alkanes varies from that of branchedalkanes in terms of the products formation. However, it is observed that the chemical kinetics of fuel oxidations for HXN, HMN and CHX are similar. For instance, H-atom abstractions on the fuel components are prevailing under fuel-lean conditions while thermal decompositions of the fuel components are more dominant under fuel-rich conditions. For stoichiometric conditions, H-atom abstractions are dominant when temperature is low whereas thermal decompositions are more prevalent when temperature is high [7,17,20] In this section, the reduced models of the fuel constituents are successfully derived using the five-stage chemical kinetic mechanism reduction scheme. The application of the reduction scheme has contributed to at least 92 % reduction in N S and 93 % reduction in N R in the reduced mechanisms as compared to the detailed mechanisms for each fuel constituents. Appropriate optimisation of selected reaction rate constants are performed to minimise the influence of eliminated species and reactions associated to the drastic mechanism reduction that has been carried out. Following that, the surrogate models are carried forward to the next section for model validations Validations of Surrogate Fuel Models in 0-D Chemical Kinetic Simulations In this section, validations of the reduced surrogate models are performed in 0-D simulations by comparing the ID timing predictions as well as species concentration profiles of important species to those of the detailed mechanisms. The test conditions applied for the mechanism validations in 0-D simulations are described in Table Validations against Detailed Models under Auto-Ignition and JSR Conditions Comparisons of ID timing predictions between the reduced and detailed mechanisms are demonstrated in Fig. 1. Here, only results for the initial pressure of 60 bar are presented. Same pattern is observed for the ID timing plots at initial pressures of 40 bar and 80 bar, 12
14 which is characterised by the S-shaped curve for ID profiles. It is observed that reasonably good agreements are achieved between the reduced and detailed mechanisms in ID predictions for each diesel fuel constituent. In this reduction work, deviations in ID are relatively evident at low and intermediate temperatures for auto-ignition conditions. Hence, further reduction will deteriorate the ID predictions as reaction pathways are more complex at this temperature range. The current results are considered satisfactory as the induced error for each prediction retains within the error tolerance of 40 % [44,51 53] In addition, capability of the reduced models for each fuel constituents in replicating concentration of important combustion products is monitored throughout this reduction work. Comparisons of the reduced and detailed mechanisms with respect to species concentration profiles for auto-ignition as well as JSR conditions are shown in Fig. 2 and Fig. 3, respectively. Only results for Ф of 1 are presented since similar temporal evolution trends in the results are obtained for both Ф of 0.5 and As shown in Fig. 2(a), there is a discrepancy between the computed fuel profiles using the detailed and reduced models of HXN. This may be partly due to the elimination of hexadecyl radical isomers in the reduced model during the reaction path analysis. As a result, the decomposition rate of the fuel species computed by the reduced HXN model is lower than that of the detailed model at the beginning of oxidation process, which eventually leads to the deviation in the temporal evolution trends for HXN. However, the influence of the deviation is not pronounced. The associated IDs as well as the mole fractions of the minor species and products computed by the reduced HXN model are still in good agreement with those of the detailed model. Furthermore, Fig. 2(b) shows that the selected species mole fractions are reasonably replicated for the HMN auto-ignition condition. On the other hand, a consistent distance between the computed species mole fraction using the reduced and detailed CHX models is observed in Fig. 2(c). This is attributed to the shorter ID calculated by the reduced 13
15 CHX model as shown earlier in Fig 1(c)(ii). Despite of this, the computed absolute species mole fractions agree with those of the detailed counterpart. Besides, satisfactory results are also obtained in species concentration predictions between the reduced and detailed models for fuel oxidations under the JSR condition, as demonstrated in Fig. 3. The findings here demonstrate an acceptable compromise in terms of mechanism size and results accuracy Validations against JSR Experimental Measurements Furthermore, the reduced mechanisms for the diesel surrogate fuel components are further validated using the JSR experimental results of HXN, HMN and CHX oxidations carried out by Ristori et al. [7], Dagaut et al. [17] and Voisin et al. [20], respectively. The open PSR model of CHEMKIN-PRO software is employed here to simulate the homogeneous condition of JSR experiments at steady state [54 56]. The validation results are depicted in Fig. 4 by comparing the computed species concentrations to the corresponding experimental measurements for fuel-oxygen mixtures, diluted by nitrogen. The selected species for comparison studies here are reactants (such as HXN, HMN, CHX, O 2 ), oxygenated products (such as CO 2, CH 2 O) as well as important products under fuel-rich region (such as C 2 H 2, C 2 H 4 and C 6 H 6 ). These species concentrations are validated to ensure that the proposed surrogate models are able to provide a reasonable representation of the kinetics of the fuel oxidations. Apart from that, concentration profiles of C 2 H 2, C 2 H 4 and C 6 H 6 are monitored as they are the major species involved in the soot formation. Both C 6 H 6 and C 2 H 2 are commonly used as soot precursors while the latter is also the soot surface growth species which is important to soot mass addition during surface growth process. On top of these, C 2 H 4 is the most abundant alkene among all the measured alkenes and it plays an important role in the formation of C 2 H 2. Thus, validation of concentration profiles of these rich combustion products is expected to aid soot formation predictions for the subsequent multi-dimensional 14
16 CFD modelling studies. As soot is mainly formed in fuel-rich condition, Ф of 1.5 is applied in the subsequent validation exercise Results in Fig. 4(a) show that the surrogate model for HXN is able to reproduce the species concentration profiles and kinetics of the fuel oxidation adequately in view of its simplified fuel chemistry. It is observed that the computed fuel profile is comparable with the experimental measurement in which the fuel concentration decreases with temperature. However, there is a minor deviation in the fuel concentration predictions as compared to the experimental measurements. The fuel concentration for HXN oxidation at high temperatures ( 1000 K) is under-predicted. Owing to the different fuel consumption rate, the formation of product species such as CH 2 O also differs from the experimental profiles across the temperature range. An opposite trend is also observed for C 2 H 4 and CH 2 O profiles between experimental data and those predicted by the reduced models, where the measured concentrations increase with increasing temperatures. This may be expected since the detailed model also predicted an opposite trend for these species, where decreasing trends are observed at high temperature region. The patterns produced by the reduced and detailed models are however consistent. Moreover, as the experimental measurement of C 2 H 2 concentration of the HXN oxidation is not available, the computed profile is added in Fig. 4 and is compared to that of the C 2 H 4. Based on the reaction path analysis of fuel oxidation routes, the formation of C 2 H 2 is significantly dependent on C 2 H 4. It is observed that both C 2 H 4 and C 2 H 2 concentrations increase with temperature when T < 1100 K. However, C 2 H 4 concentration starts to decrease thereafter while C 2 H 2 concentration continues to rise. This could be attributed to the consumption of C 2 H 4 which leads to the formation of C 2 H 2 [57]. In addition, the species concentration profiles predicted by the reduced mechanism are also compared with those of the detailed mechanism. Consistency in species profile trends is 15
17 observed between the computed results using the reduced and detailed mechanisms, as demonstrated in Fig. 4(a) Similar to the HXN oxidation, Fig. 4(b) demonstrates that the HMN concentration decreases when ambient temperature increases. Besides, it is also observed that the computed fuel concentration using the reduced chemistry is over-predicted at 800 K < T < 1000 K as compared to the experimental measurements. The deviations between the computed results and the experimental measurements are within one order of magnitude in the absolute values. In spite of this, the species profiles for the resulting product species such as C 2 H 2, C 2 H 4 and C 2 H 6 are seen to be consistent and identical. Additionally, similar temporal evolution trends are observed between the reduced and detailed mechanisms Furthermore, based on the results obtained for CHX oxidation in Fig. 4(c), decreasing trend in fuel profile is obtained and fuel concentrations at T > 850 K are under-predicted. Overall agreement is achieved between the species concentration predictions and the experimental measurements. Besides these, the species profile trends predicted by the reduced mechanism are consistent with those of the detailed mechanism Although variation of the computed concentrations could reach as high as one order of magnitude as compared to the JSR experimental measurements, the results of the predicted species concentrations are deemed acceptable in view of their simplified fuel chemistries. The overall agreements between the experimental and predicted species profiles for HXN, HMN and CHX are achieved and these fuel constituent models are henceforth used in Part II to construct multi-component diesel surrogate models Conclusions 16
18 In this study, reduced models of branched- (HMN) and cyclo- (CHX) alkanes are developed using the five-stage chemical kinetic mechanism reduction scheme [39,11] formulated in the previous work. With the implementation of the reduction scheme, reductions of 92 % and 93 % in terms of N S and N R, respectively, are achieved in comparison to the detailed mechanisms for both fuel constituents. As a result, a reduced HMN mechanism with 89 species and a reduced CHX mechanism with 80 species are developed. Subsequently, both the aforementioned constituent mechanisms, together with the reduced HXN mechanism derived in the previous work are comprehensively validated in 0-D chemical kinetic simulations under a wide range of shock tube and JSR conditions in terms of ID timing and species concentration predictions. The overall ID timings and species concentrations at the test conditions agree well with those calculated using the respective detailed mechanisms. The maximum deviations from the detailed models in ID timing predictions are maintained to within 40 %. Apart from that, trends of the species concentration profiles computed by the fuel constituent reduced models are also retained as compared to those of the detailed models for both auto-ignition and JSR conditions. The relative errors between the computations of the reduced and detailed models remain 40 % throughout the test conditions. In addition, the fidelity of the reduced models is further assessed using the experimental data of respective fuel oxidations in a JSR. The experimental species concentration profiles for each fuel constituents are adequately reproduced with maximum deviations of one order of magnitude in the absolute values The construction of the reduced models for each diesel fuel constituents in this work is an essential prerequisite for the developmental work of multi-component diesel surrogate fuel models presented in Part II. These diesel fuel constituent surrogate models are also ready to be used independently for other CFD applications such as HCCI and direct injection engine combustion simulations [10,31]. It is worth mentioning that, the reduced models are 17
19 constructed specifically for engine-like operating conditions such that the chemistry can be minimised and a practical level of computational efficiency in multi-dimensional CFD simulations can be achieved. Hence, these mechanisms have to be used with care when they are applied for other applications. 411 Acknowledgements The Ministry of Higher Education Malaysia is acknowledged for the financial support towards this project under the Fundamental Research Grant Scheme (FRGS) F The work at Technical University of Denmark is supported by Innovation Fund Denmark and MAN Diesel & Turbo A/S through the RADIADE project References [1] Farrell JT, Cernansky NP, Dryer FL, Law CK, Friend DG, Hergart CA, et al. Development of an Experimental Database and Kinetic Models for Surrogate Diesel Fuels. SAE Technical Paper ; [2] Morrison RD, Murphy BL. Environmental Forensics: Contaminant Specific Guide. Elsevier; [3] Roberts JD, Caserio MC. Basic Principles of Organic Chemistry. WA Benjamin, Inc.; [4] Lu T, Law CK. Toward Accommodating Realistic Fuel Chemistry in Large-Scale Computations. Prog Energy Combust Sci 2009;35: [5] Meijer M. Characterization of n-heptane as a Single Component Diesel Surrogate Fuel. Technical University of Eindhoven Automotive Technology, Graduation Thesis; [6] Westbrook CK, Pitz WJ, Herbinet O, Curran HJ, Silke EJ. A Comprehensive Detailed Chemical Kinetic Reaction Mechanism for Combustion of n-alkane Hydrocarbons from n-octane to n-hexadecane. Combust Flame 2009;156: [7] Ristori A, Dagaut P, Cathonnet M. The Oxidation of n-hexadecane: Experimental and Detailed Kinetic Modeling. Combust Flame 2001;125: [8] Chaos M, Kazakov A, Dryer FL, Zhao Z, Zeppieri SP. High Temperature Compact Mechanism Development for Large Alkanes: n-hexadecane. In: 6th Int. Conf. Chem. Kinet., [9] Fournet R, Battin-Leclerc F, Glaude PA, Judenherc B, Warth V, Come GM, et al. The Gas-Phase Oxidation of n-hexadecane. Int J Chem Kinet 2001;33: [10] Liang L, Puduppakkam K, Meeks E. Towards Using Realistic Chemical Kinetics in Multidimensional CFD. In: 19th Int. Multidimens. Engine Model. User s Gr. Meet., [11] Poon HM, Ng HK, Gan S, Pang KM, Schramm J. Development and Validation of Chemical Kinetic Mechanism Reduction Scheme for Large-Scale Mechanisms. SAE Int J Fuels Lubr 2014;7:
20 [12] Galle J, Sebastian V. Influence of Diesel Surrogates on the Behavior of Simplified Spray Models. Proc FISITA 2012 World Automot Congr 2013;189: [13] Pitz WJ, Cernansky NP, Dryer FL, Egolfopoulos FN, Farrell JT, Friend DG, et al. Development of an Experimental Database and Chemical Kinetic Models for Surrogate Gasoline Fuels. SAE Technical Paper ; [14] Westbrook CK, Pitz WJ, Mehl M, Curran HJ. Detailed Chemical Kinetic Reaction Mechanisms for Primary Reference Fuels for Diesel Cetane Number and Spark- Ignition Octane Number. Proc Combust Inst 2011;33: [15] Agosta A, Cernansky NP, Miller DL, Faravelli T, Ranzi E. Reference Components of Jet Fuels: Kinetic Modeling and Experimental Results. Exp Therm Fluid Sci 2004;28: [16] Lenhert DB, Cernansky NP, Miller DL. The Oxidation of Large cular Weight Hydrocarbons in a Pressurized Flow Reactor. In: 4th Jt. Meet. US Sect. Combust. Inst., [17] Dagaut P, Hadj-Ali K. Chemical Kinetic Study of the Oxidation of Isocetane (2, 2, 4, 4, 6, 8, 8-Heptamethylnonane) in a Jet-Stirred Reactor: Experimental and Modeling. Energy & Fuels 2009;19: [18] Oehlschlaeger MA, Steinberg J, Westbrook CK, Pitz WJ. The Autoignition of iso- Cetane at High to Moderate Temperatures and Elevated Pressures: Shock Tube Experiments and Kinetic Modeling. Combust Flame 2009;156: [19] Curran HJ, Gaffuri P, Pitz WJ, Westbrook CK. A Comprehensive Modeling Study of iso-octane Oxidation. Combust Flame 2002;129: [20] Voisin D, Marchal A, Reuillon M, Boettner JC, Cathonnet M. Experimental and Kinetic Modeling Study of Cyclohexane Oxidation in a JSR at High Pressure. Combust Sci Technol 1998;138: [21] Silke EJ, Pitz WJ, Westbrook CK, Ribaucour M. Detailed Chemical Kinetic Modeling of Cyclohexane Oxidation. J Phys Chem A 2007;111: [22] Daley SM, Berkowitz AM, Oehlschlaeger MA. A Shock Tube Study of Cyclopentane and Cyclohexane Ignition at Elevated Pressures. Int J Chem Kinet 2008;40: [23] El Bakali A, Braun-Unkhoff M, Dagaut P, Frank P, Cathonnet M. Detailed Kinetic Reaction Mechanism for Cyclohexane Oxidation at Pressure Up to Ten Atmospheres. Proc Combust Inst 2000;28: [24] Law ME, Westmoreland PR, Cool TA, Wang J, Hansen N, Taatjes CA, et al. Benzene Precursors and Formation Routes in a Stoichiometric Cyclohexane Flame. Proc Combust Inst 2007;31 I: [25] Tsang W. Thermal Decomposition of Cyclopentane and Related Compounds. Int J Chem Kinet 1978;10: [26] Tsang W. Thermal Stability of Cyclohexane and 1-Hexene. Int J Chem Kinet 1978;10: [27] Sirjean B, Buda F, Hakka H, Glaude PA, Fournet R, Warth V, et al. The Autoignition of Cyclopentane and Cyclohexane in a Shock Tube. Proc Combust Inst 2007;31 I: [28] Simon V, Simon Y, Scacchi G, Baronnet F. Étude Expérimentale et Modélisation des Réactions d Oxydation du n-pentane et du Cyclopentane. Can J Chem 1997;75: [29] Davis SG, Law CK. Determination of and Fuel Structure Effects on Laminar Flame Speeds of C1 to C8 Hydrocarbons. Combust Sci Technol 1998;140: [30] Billaud F, Chaverot P, Berthelin M, Freund E. Thermal Decomposition of Cyclohexane at Approximately 810 Degree C. Ind Eng Chem Res 1988;27: [31] Ra Y, Reitz RD. A Combustion Model for IC Engine Combustion Simulations with 19
21 Multi-Component Fuels. Combust Flame 2011;158: [32] Griffiths JF, Piazzesi R, Sazhina EM, Sazhin SS, Glaude PA, Heikal MR. CFD Modelling of Cyclohexane Auto-Ignition in an RCM. Fuel 2012;96: [33] Golovitchev VI, Bergman M, Montorsi L. CFD Modeling of Diesel Oil and DME Performance in a Two-Stroke Free Piston Engine. Combust Sci Technol 2007;179: [34] Wang H, Jiao Q, Yao M, Yang B, Qiu L, Reitz RD. Development of an n- Heptane/Toluene/Polyaromatic Hydrocarbon Mechanism and its Application for Combustion and Soot Prediction. Int J Engine Res 2013;14: [35] Ranzi E, Frassoldati A, Stagni A, Pelucchi M, Cuoci A, Faravelli T. Reduced Kinetic Schemes of Complex Reaction Systems: Fossil and Biomass-Derived Transportation Fuels. Int J Chem Kinet 2014;46: [36] Pitz WJ, Seiser R, J. W. Bozzelli, K. Seshadri, Chen CJ, Costa ID, et al. Chemical Kinetic Study of Toluene Oxidation Under Premixed and Nonpremixed Conditions. Lawrence Livermore Natl Lab 2003:1 20. [37] Wang H, Yao M, Reitz RD. Development of a Reduced Primary Reference Fuel Mechanism for Internal Combustion Engine Combustion Simulations. Energy & Fuels 2013;27: [38] Chang Y, Jia M, Li Y, Liu Y, Xie M, Wang H, et al. Development of a Skeletal Mechanism for Diesel Surrogate Fuel by Using a Decoupling Methodology. Combust Flame 2015;162: [39] Poon HM, Ng HK, Gan S, Pang KM, Schramm J. Evaluation and Development of Chemical Kinetic Mechanism Reduction Scheme for Biodiesel and Diesel Fuel Surrogates. SAE Int J Fuels Lubr 2013;6: [40] Lu T, Law CK. On the Applicability of Directed Relation Graphs to the Reduction of Reaction Mechanisms. Combust Flame 2006;146: [41] Pepiot P, Pitsch H. Systematic Reduction of Large Chemical Mechanisms. In: 4th Jt. Meet. U.S. Sect. Combust. Inst., 2005, p [42] Dijkstra EW. A Note on Two Problems in Connexion with Graphs. Numer Math 1959: [43] Lu T, Law CK. Strategies for Mechanism Reduction for Large Hydrocarbons: n- Heptane. Combust Flame 2008;154: [44] Brakora JL, Ra Y, Reitz RD. Combustion Model for biodiesel-fueled Engine Simulations Using Realistic Chemistry and Physical Properties. SAE Int J Engines 2011;4: [45] Lu T, Law CK. A Directed Relation Graph Method for Mechanism Reduction. Proc Combust Inst 2005;30: [46] Lu T, Law CK. Linear Time Reduction of Large Kinetic Mechanisms with Directed Relation Graph: n-heptane and iso-octane. Combust Flame 2006;144: [47] Cheng X, Ng HK, Gan S, Ho JH, Pang KM. Development and Validation of a Generic Reduced Chemical Kinetic Mechanism for CFD Spray Combustion Modelling of Biodiesel Fuels. Combust Flame 2015;162: [48] Wang H, Yao M, Yue Z, Jia M, Reitz RD. A Reduced Toluene Reference Fuel Chemical Kinetic Mechanism for Combustion and Polycyclic-Aromatic Hydrocarbon Predictions. Combust Flame 2015;162: [49] Pang KM, Ng HK, Gan S. Development of an Integrated Reduced Fuel Oxidation and Soot Precursor Formation Mechanism for CFD Simulations of Diesel Combustion. Fuel 2011;90: [50] Le MK, Kook S. Injection Pressure Effects on the Flame Development in a Light-Duty Optical Diesel Engine. SAE Int J Engines 2015;8:
22 [51] Niemeyer KE, Sung C-J, Raju MP. Skeletal Mechanism Generation for Surrogate Fuels Using Directed Relation Graph with Error Propagation and Sensitivity Analysis. Combust Flame 2010;157: [52] Yang J, Johansson M, Naik C, Puduppakkam K, Golovitchev V, Meeks E. 3D CFD Modeling of a Biodiesel-Fueled Diesel Engine Based on a Detailed Chemical Mechanism. SAE Technical Paper ; [53] Luo Z, Plomer M, Lu T, Som S, Longman DE, Sarathy SM, et al. A Reduced Mechanism for Biodiesel Surrogates for Compression Ignition Engine Applications. Fuel 2012;99: [54] Battin-Leclerc F, Simmie JM, Blurock E. Cleaner Combustion: Developing Detailed Chemical Kinetic Models. Springer Science & Business Media; [56] Dagaut P, Cathonnet M, Rouan JP, Foulatier R, Quilgars A, Boettner JC, et al. A Jet- Stirred Reactor for Kinetic Studies of Homogeneous Gas-Phase Reactions at Pressures Up to Ten Atmospheres ( 1 MPa). J Phys E: Scientific Instruments 1986;19: [57] Veloo PS, Dagaut P, Togbé C, Dayma G, Sarathy SM, Westbrook CK, et al. Experimental and Modeling Study of the Oxidation of n- and iso-butanal. Combust Flame 2013;160:
23 ID Timing (s) ID Timing (s) ID Timing (s) (a)(i) Ф = 0.5 (a)(ii) Ф = 1 (a)(iii) Ф = 2 Fig. 1 Computed ID of (a) HXN, (b) HMN and (c) CHX calculated by respective detailed and reduced mechanisms, with initial pressure of 60 bar and Ф of (i) 0.5, (ii) 1.0, (iii) 2.0. (b)(i) Ф = 0.5 (c)(i) Ф = /T (1/K) (b)(ii) Ф = 1 (c)(ii) Ф = /T (1/K) (b)(iii) Ф = 2 (c)(iii) Ф = /T (1/K) Detailed Mechanism Reduced Mechanism 1
24 2E-2 3E-1 5E-3 HXN OH 2E-1 O 2 2E-2 2E-3 C 2 H 2 5E-2 CO 2 HO 2 4E-4 8E-4 Time (s) 4E-4 8E-4 Time (s) 2E-2 (a) 4E-4 8E-4 Time (s) 3E-1 5E-3 HMN OH 2E-1 O 2 4E-3 2E-3 C 2 H 2 5E-2 CO 2 5E-4 HO 2 2E-3 4E-3 2E-3 4E-3 Time (s) Time (s) (b) 3E-2 2E-2 CHX 4E-3 OH 5E-3 2E-3 4E-3 Time (s) 3E-1 2E-1 O 2 2E-3 C 2 H 2 5E-2 CO 2 5E-4 HO 2 4E-3 8E-3 4E-3 8E-3 4E-3 8E-3 Time (s) Time (s) Time (s) (c) Detailed Mechanism Reduced Mechanism Fig. 2 Computed species profiles for (a) HXN, (b) HMN and (c) CHX combustions under auto-ignition condition, with initial pressure of 60 bar, initial temperature of 950 K and Ф of 1. 2
25 1E+0 HXN O 2 1E-8 OH 1E-8 HO E+0 HMN 1E+0 CO E+0 (a) O 2 0 1E+0 CO E-7 1E-9 OH 1E-8 HO CHX 1E-8 HO E+0 CO E+0 1E+0 (c) Detailed Mechanism Reduced Mechanism Fig. 3 Computed species profiles of (a) HXN, (b) HMN and (c) CHX oxidations under JSR condition as a function of temperature, with initial pressure of 60 bar and Ф of 1.0. (b) O 2 1E-8 0 CO E+0 CO OH 1E-8 C 6 H 5 CH
26 HXN O₂ C₂H₄ CO₂ 1E HMN 1E-7 C₂H₄ CHX C₂H₄ 1E E-7 (c) Experimental Measurements Detailed Mechanism Reduced Mechanism Fig. 4 Computed and experimental species mole fractions obtained from the oxidation of (a) 0.03 % HXN (pressure = 1 atm, Ф = 1.5, residence time = 70 ms), (b) 0.07 % HMN (pressure = 10 atm, Ф = 2, residence time of 1 s), and (c) 0.1 % CHX (pressure = 10 atm, Ф = 1.5, residence time = 0.5 s) in a JSR. (a) (b) C₂H₂ CH₂O C₂H₆ C₂H₂ CO₂ 1E-7 C₂H₂ 1E-9 CO₂ 1E E-7 CH₂O E-7 C₆H₆ 1E-9 CH₂O 1E
Confirmation of paper submission
 Dr. Marina Braun-Unkhoff Institute of Combustion Technology DLR - German Aerospace Centre Pfaffenwaldring 30-40 70569 Stuttgart 28. Mai 14 Confirmation of paper submission Name: Email: Co-author: 2nd co-author:
Dr. Marina Braun-Unkhoff Institute of Combustion Technology DLR - German Aerospace Centre Pfaffenwaldring 30-40 70569 Stuttgart 28. Mai 14 Confirmation of paper submission Name: Email: Co-author: 2nd co-author:
Flame Studies of Small Hydrocarbons and Oxygenated Fuels
 Flame Studies of Small Hydrocarbons and Oxygenated Fuels Peter Veloo, Yang L. Wang, Okjoo Park, Qiayo Feng, Aydin Jalali, Roe Burrell, Adam Fincham, Charles K. Westbrook, Fokion N. Egolfopoulos Department
Flame Studies of Small Hydrocarbons and Oxygenated Fuels Peter Veloo, Yang L. Wang, Okjoo Park, Qiayo Feng, Aydin Jalali, Roe Burrell, Adam Fincham, Charles K. Westbrook, Fokion N. Egolfopoulos Department
Study on cetane number dependence of. with a controlled temperature profile
 3 August 2012 (5E06) The 34th International Symposium on Combustion Study on cetane number dependence of diesel surrogates/air weak flames in a micro flow reactor with a controlled temperature profile
3 August 2012 (5E06) The 34th International Symposium on Combustion Study on cetane number dependence of diesel surrogates/air weak flames in a micro flow reactor with a controlled temperature profile
Marc ZELLAT, Driss ABOURI, Thierry CONTE and Riyad HECHAICHI CD-adapco
 16 th International Multidimensional Engine User s Meeting at the SAE Congress 2006,April,06,2006 Detroit, MI RECENT ADVANCES IN SI ENGINE MODELING: A NEW MODEL FOR SPARK AND KNOCK USING A DETAILED CHEMISTRY
16 th International Multidimensional Engine User s Meeting at the SAE Congress 2006,April,06,2006 Detroit, MI RECENT ADVANCES IN SI ENGINE MODELING: A NEW MODEL FOR SPARK AND KNOCK USING A DETAILED CHEMISTRY
Simulation of single diesel droplet evaporation and combustion process with a unified diesel surrogate
 ILASS-Americas 29th Annual Conference on Liquid Atomization and Spray Systems, Atlanta, GA, May 2017 Simulation of single diesel droplet evaporation and combustion process with a unified diesel surrogate
ILASS-Americas 29th Annual Conference on Liquid Atomization and Spray Systems, Atlanta, GA, May 2017 Simulation of single diesel droplet evaporation and combustion process with a unified diesel surrogate
A Rapid Compression Study of the Butanol Isomers at Elevated Pressure
 7 th US National Technical Meeting of the Combustion Institute Hosted by the Georgia Institute of Technology, Atlanta, GA March -23, 11 A Rapid Compression Study of the Butanol Isomers at Elevated Pressure
7 th US National Technical Meeting of the Combustion Institute Hosted by the Georgia Institute of Technology, Atlanta, GA March -23, 11 A Rapid Compression Study of the Butanol Isomers at Elevated Pressure
The Ignition of C 7 -C 16 Normal and Branched Alkanes at Elevated Pressures
 The Ignition of C 7 -C 16 Normal and Branched Alkanes at Elevated Pressures Matthew A. Oehlschlaeger*, Hsi-Ping S. Shen, Justin Steinberg, and Jeremy Vanderover Department of Mechanical, Aerospace, and
The Ignition of C 7 -C 16 Normal and Branched Alkanes at Elevated Pressures Matthew A. Oehlschlaeger*, Hsi-Ping S. Shen, Justin Steinberg, and Jeremy Vanderover Department of Mechanical, Aerospace, and
PDF-based simulations of in-cylinder combustion in a compression-ignition engine
 Paper # 070IC-0192 Topic: Internal Combustion Engines 8 th US National Combustion Meeting Organized by the Western States Section of the Combustion Institute and hosted by the University of Utah May 19-22,
Paper # 070IC-0192 Topic: Internal Combustion Engines 8 th US National Combustion Meeting Organized by the Western States Section of the Combustion Institute and hosted by the University of Utah May 19-22,
DARS FUEL MODEL DEVELOPMENT
 DARS FUEL MODEL DEVELOPMENT DARS Products (names valid since October 2012) DARS 0D & 1D tools Old name: DARS Basic DARS Reactive Flow Models tools for 3D/ CFD calculations DARS Fuel New! Advanced fuel
DARS FUEL MODEL DEVELOPMENT DARS Products (names valid since October 2012) DARS 0D & 1D tools Old name: DARS Basic DARS Reactive Flow Models tools for 3D/ CFD calculations DARS Fuel New! Advanced fuel
Prediction of Physical Properties and Cetane Number of Diesel Fuels and the Effect of Aromatic Hydrocarbons on These Entities
 [Regular Paper] Prediction of Physical Properties and Cetane Number of Diesel Fuels and the Effect of Aromatic Hydrocarbons on These Entities (Received March 13, 1995) The gross heat of combustion and
[Regular Paper] Prediction of Physical Properties and Cetane Number of Diesel Fuels and the Effect of Aromatic Hydrocarbons on These Entities (Received March 13, 1995) The gross heat of combustion and
RECENT PROGRESS IN THE DEVELOPMENT OF DIESEL SURROGATE FUELS
 CRC Report No. AVFL-18a RECENT PROGRESS IN THE DEVELOPMENT OF DIESEL SURROGATE FUELS December 2009 COORDINATING RESEARCH COUNCIL, INC. 3650 MANSELL ROAD SUITE 140 ALPHARETTA, GA 30022 The Coordinating
CRC Report No. AVFL-18a RECENT PROGRESS IN THE DEVELOPMENT OF DIESEL SURROGATE FUELS December 2009 COORDINATING RESEARCH COUNCIL, INC. 3650 MANSELL ROAD SUITE 140 ALPHARETTA, GA 30022 The Coordinating
Homogeneous Charge Compression Ignition combustion and fuel composition
 Loughborough University Institutional Repository Homogeneous Charge Compression Ignition combustion and fuel composition This item was submitted to Loughborough University's Institutional Repository by
Loughborough University Institutional Repository Homogeneous Charge Compression Ignition combustion and fuel composition This item was submitted to Loughborough University's Institutional Repository by
Jet fuels and Fischer-Tropsch fuels: Surrogate definition and chemical kinetic modeling
 Paper # 070RK-0273 Topic: Reaction Kinetics 8 th US National Combustion Meeting Organized by the Western States Section of the Combustion Institute and hosted by the University of Utah May 9-22, 203. Jet
Paper # 070RK-0273 Topic: Reaction Kinetics 8 th US National Combustion Meeting Organized by the Western States Section of the Combustion Institute and hosted by the University of Utah May 9-22, 203. Jet
CFD Combustion Models for IC Engines. Rolf D. Reitz
 CFD Combustion Models for IC Engines Rolf D. Reitz Engine Research Center University of Wisconsin-Madison ERC Symposium, June 7, 27 http://www.cae.wisc.edu/~reitz Combustion and Emission Models at the
CFD Combustion Models for IC Engines Rolf D. Reitz Engine Research Center University of Wisconsin-Madison ERC Symposium, June 7, 27 http://www.cae.wisc.edu/~reitz Combustion and Emission Models at the
Flow Reactors for Validation Data Base Development
 Flow Reactors for Validation Data Base Development Frederick L. Dryer Mechanical and Aerospace Engineering Princeton University 27 AFOSR MURI Kick-Off Meeting Generation of Comprehensive Surrogate Kinetic
Flow Reactors for Validation Data Base Development Frederick L. Dryer Mechanical and Aerospace Engineering Princeton University 27 AFOSR MURI Kick-Off Meeting Generation of Comprehensive Surrogate Kinetic
Introduction to combustion
 Introduction to combustion EEN-E005 Bioenergy 1 017 D.Sc (Tech) ssi Kaario Motivation Why learn about combustion? Most of the energy in the world, 70-80%, is produced from different kinds of combustion
Introduction to combustion EEN-E005 Bioenergy 1 017 D.Sc (Tech) ssi Kaario Motivation Why learn about combustion? Most of the energy in the world, 70-80%, is produced from different kinds of combustion
Marc ZELLAT, Driss ABOURI and Stefano DURANTI CD-adapco
 17 th International Multidimensional Engine User s Meeting at the SAE Congress 2007,April,15,2007 Detroit, MI RECENT ADVANCES IN DIESEL COMBUSTION MODELING: THE ECFM- CLEH COMBUSTION MODEL: A NEW CAPABILITY
17 th International Multidimensional Engine User s Meeting at the SAE Congress 2007,April,15,2007 Detroit, MI RECENT ADVANCES IN DIESEL COMBUSTION MODELING: THE ECFM- CLEH COMBUSTION MODEL: A NEW CAPABILITY
Fundamental Kinetics Database Utilizing Shock Tube Measurements
 Fundamental Kinetics Database Utilizing Shock Tube Measurements Volume 1: Ignition Delay Time Measurements D. F. Davidson and R. K. Hanson Mechanical Engineering Department Stanford University, Stanford
Fundamental Kinetics Database Utilizing Shock Tube Measurements Volume 1: Ignition Delay Time Measurements D. F. Davidson and R. K. Hanson Mechanical Engineering Department Stanford University, Stanford
INVESTIGATION OF AUTO-IGNITION OF HEPTANE-CNG MIXTURE IN HCCI ENGINE. Firmansyah. Universiti Teknologi PETRONAS
 INVESTIGATION OF AUTO-IGNITION OF HEPTANE-CNG MIXTURE IN HCCI ENGINE Firmansyah Universiti Teknologi PETRONAS OUTLINE INTRODUCTION OBJECTIVES METHODOLOGY RESULTS and DISCUSSIONS CONCLUSIONS HCCI DUALFUELCONCEPT
INVESTIGATION OF AUTO-IGNITION OF HEPTANE-CNG MIXTURE IN HCCI ENGINE Firmansyah Universiti Teknologi PETRONAS OUTLINE INTRODUCTION OBJECTIVES METHODOLOGY RESULTS and DISCUSSIONS CONCLUSIONS HCCI DUALFUELCONCEPT
Perfectly Stirred Reactor Network Modeling of NOx and CO Emissions from a Gas Turbine Combustor with Water Addition
 Perfectly Stirred Reactor Network Modeling of NOx and CO Emissions from a Gas Turbine Combustor with Water Addition Abstract For Submission in Partial Fulfillment of the UTSR Fellowship Program Andrew
Perfectly Stirred Reactor Network Modeling of NOx and CO Emissions from a Gas Turbine Combustor with Water Addition Abstract For Submission in Partial Fulfillment of the UTSR Fellowship Program Andrew
Module8:Engine Fuels and Their Effects on Emissions Lecture 36:Hydrocarbon Fuels and Quality Requirements FUELS AND EFFECTS ON ENGINE EMISSIONS
 FUELS AND EFFECTS ON ENGINE EMISSIONS The Lecture Contains: Transport Fuels and Quality Requirements Fuel Hydrocarbons and Other Components Paraffins Cycloparaffins Olefins Aromatics Alcohols and Ethers
FUELS AND EFFECTS ON ENGINE EMISSIONS The Lecture Contains: Transport Fuels and Quality Requirements Fuel Hydrocarbons and Other Components Paraffins Cycloparaffins Olefins Aromatics Alcohols and Ethers
Numerical Study on the Combustion and Emission Characteristics of Different Biodiesel Fuel Feedstocks and Blends Using OpenFOAM
 Numerical Study on the Combustion and Emission Characteristics of Different Biodiesel Fuel Feedstocks and Blends Using OpenFOAM Harun M. Ismail 1, Xinwei Cheng 1, Hoon Kiat Ng 1, Suyin Gan 1 and Tommaso
Numerical Study on the Combustion and Emission Characteristics of Different Biodiesel Fuel Feedstocks and Blends Using OpenFOAM Harun M. Ismail 1, Xinwei Cheng 1, Hoon Kiat Ng 1, Suyin Gan 1 and Tommaso
Nomenclature. I. Introduction. Research Assistant, Department of Mechanical Engineering University of Connecticut, Student Member AIAA.
 This work is licensed under the Creative Commons Autoignition of Butanol Isomers at Low to Intermediate Temperature and Elevated Pressure Bryan Weber, Kamal Kumar 2 and Chih-Jen Sung 3 University of Connecticut,
This work is licensed under the Creative Commons Autoignition of Butanol Isomers at Low to Intermediate Temperature and Elevated Pressure Bryan Weber, Kamal Kumar 2 and Chih-Jen Sung 3 University of Connecticut,
INVESTIGATION OF AUTO-IGNITION OF HEPTANE-CNG MIXTURE IN HCCI ENGINE
 INVESTIGATION OF AUTO-IGNITION OF HEPTANE-CNG MIXTURE IN HCCI ENGINE Firmansyah a, A. Rashid. A. Aziz b Universiti Teknologi PETRONAS Perak darul ridzuan, 31750, Malaysia firmansyah@petronas.com.my, rashid@petronas.com.my
INVESTIGATION OF AUTO-IGNITION OF HEPTANE-CNG MIXTURE IN HCCI ENGINE Firmansyah a, A. Rashid. A. Aziz b Universiti Teknologi PETRONAS Perak darul ridzuan, 31750, Malaysia firmansyah@petronas.com.my, rashid@petronas.com.my
Thermo-Kinetic Model to Predict Start of Combustion in Homogeneous Charge Compression Ignition Engine
 Thermo-Kinetic Model to Predict Start of Combustion in Homogeneous Charge Compression Ignition Engine Harshit Gupta and J. M. Malliarjuna Abstract Now-a-days homogeneous charge compression ignition combustion
Thermo-Kinetic Model to Predict Start of Combustion in Homogeneous Charge Compression Ignition Engine Harshit Gupta and J. M. Malliarjuna Abstract Now-a-days homogeneous charge compression ignition combustion
REPORT DOCUMENTATION PAGE
 REPORT DOCUMENTATION PAGE Form Approved OMB NO. 0704-0188 The public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instructions,
REPORT DOCUMENTATION PAGE Form Approved OMB NO. 0704-0188 The public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instructions,
EFFECT OF INJECTION ORIENTATION ON EXHAUST EMISSIONS IN A DI DIESEL ENGINE: THROUGH CFD SIMULATION
 EFFECT OF INJECTION ORIENTATION ON EXHAUST EMISSIONS IN A DI DIESEL ENGINE: THROUGH CFD SIMULATION *P. Manoj Kumar 1, V. Pandurangadu 2, V.V. Pratibha Bharathi 3 and V.V. Naga Deepthi 4 1 Department of
EFFECT OF INJECTION ORIENTATION ON EXHAUST EMISSIONS IN A DI DIESEL ENGINE: THROUGH CFD SIMULATION *P. Manoj Kumar 1, V. Pandurangadu 2, V.V. Pratibha Bharathi 3 and V.V. Naga Deepthi 4 1 Department of
Progress in Predicting Soot Particle Numbers in CFD Simulations of GDI and Diesel Engines
 International Multidimensional Engine Modeling User's Group Meeting April 20, 2015, Detroit, Michigan Progress in Predicting Soot Particle Numbers in CFD Simulations of GDI and Diesel Engines Abstract
International Multidimensional Engine Modeling User's Group Meeting April 20, 2015, Detroit, Michigan Progress in Predicting Soot Particle Numbers in CFD Simulations of GDI and Diesel Engines Abstract
Foundations of Thermodynamics and Chemistry. 1 Introduction Preface Model-Building Simulation... 5 References...
 Contents Part I Foundations of Thermodynamics and Chemistry 1 Introduction... 3 1.1 Preface.... 3 1.2 Model-Building... 3 1.3 Simulation... 5 References..... 8 2 Reciprocating Engines... 9 2.1 Energy Conversion...
Contents Part I Foundations of Thermodynamics and Chemistry 1 Introduction... 3 1.1 Preface.... 3 1.2 Model-Building... 3 1.3 Simulation... 5 References..... 8 2 Reciprocating Engines... 9 2.1 Energy Conversion...
Shock-tube study of the addition effect of CF 2 BrCl on the ignition of light hydrocarbons
 25 th ICDERS August 2 7, 2015 Leeds, UK Shock-tube study of the addition effect of CF 2 BrCl on the ignition of light hydrocarbons O. Mathieu, C. Gregoire, and E. L. Petersen Texas A&M University, Department
25 th ICDERS August 2 7, 2015 Leeds, UK Shock-tube study of the addition effect of CF 2 BrCl on the ignition of light hydrocarbons O. Mathieu, C. Gregoire, and E. L. Petersen Texas A&M University, Department
INFLUENCE OF THE NUMBER OF NOZZLE HOLES ON THE UNBURNED FUEL IN DIESEL ENGINE
 INFLUENCE OF THE NUMBER OF NOZZLE HOLES ON THE UNBURNED FUEL IN DIESEL ENGINE 1. UNIVERSITY OF RUSE, 8, STUDENTSKA STR., 7017 RUSE, BULGARIA 1. Simeon ILIEV ABSTRACT: The objective of this paper is to
INFLUENCE OF THE NUMBER OF NOZZLE HOLES ON THE UNBURNED FUEL IN DIESEL ENGINE 1. UNIVERSITY OF RUSE, 8, STUDENTSKA STR., 7017 RUSE, BULGARIA 1. Simeon ILIEV ABSTRACT: The objective of this paper is to
STATE OF THE ART OF PLASMATRON FUEL REFORMERS FOR HOMOGENEOUS CHARGE COMPRESSION IGNITION ENGINES
 Bulletin of the Transilvania University of Braşov Vol. 3 (52) - 2010 Series I: Engineering Sciences STATE OF THE ART OF PLASMATRON FUEL REFORMERS FOR HOMOGENEOUS CHARGE COMPRESSION IGNITION ENGINES R.
Bulletin of the Transilvania University of Braşov Vol. 3 (52) - 2010 Series I: Engineering Sciences STATE OF THE ART OF PLASMATRON FUEL REFORMERS FOR HOMOGENEOUS CHARGE COMPRESSION IGNITION ENGINES R.
THE USE OF Φ-T MAPS FOR SOOT PREDICTION IN ENGINE MODELING
 THE USE OF ΦT MAPS FOR SOOT PREDICTION IN ENGINE MODELING Arturo de Risi, Teresa Donateo, Domenico Laforgia Università di Lecce Dipartimento di Ingegneria dell Innovazione, 731 via Arnesano, Lecce Italy
THE USE OF ΦT MAPS FOR SOOT PREDICTION IN ENGINE MODELING Arturo de Risi, Teresa Donateo, Domenico Laforgia Università di Lecce Dipartimento di Ingegneria dell Innovazione, 731 via Arnesano, Lecce Italy
Numerical Study of Multi-Component Spray Combustion with a Discrete Multi- Component Fuel Model
 Numerical Study of Multi-Component Spray Combustion with a Discrete Multi- Component Fuel Model Y. Ra, and R. D. Reitz Engine Research Center, University of Wisconsin-Madison Madison, Wisconsin 53706 USA
Numerical Study of Multi-Component Spray Combustion with a Discrete Multi- Component Fuel Model Y. Ra, and R. D. Reitz Engine Research Center, University of Wisconsin-Madison Madison, Wisconsin 53706 USA
Emissions predictions for Diesel engines based on chemistry tabulation
 Emissions predictions for Diesel engines based on chemistry tabulation C. Meijer, F.A. Tap AVL Dacolt BV (The Netherlands) M. Tvrdojevic, P. Priesching AVL List GmbH (Austria) 1. Introduction It is generally
Emissions predictions for Diesel engines based on chemistry tabulation C. Meijer, F.A. Tap AVL Dacolt BV (The Netherlands) M. Tvrdojevic, P. Priesching AVL List GmbH (Austria) 1. Introduction It is generally
Autoignition Studies of Alternative Fuels
 Autoignition Studies of Alternative Fuels Chih-Jen (Jackie) Sung Department of Mechanical Engineering University of Connecticut Prepared for Second Annual CEFRC Conference Princeton, NJ August 17, 2011
Autoignition Studies of Alternative Fuels Chih-Jen (Jackie) Sung Department of Mechanical Engineering University of Connecticut Prepared for Second Annual CEFRC Conference Princeton, NJ August 17, 2011
Maximizing Engine Efficiency by Controlling Fuel Reactivity Using Conventional and Alternative Fuels. Sage Kokjohn
 Maximizing Engine Efficiency by Controlling Fuel Reactivity Using Conventional and Alternative Fuels Sage Kokjohn Acknowledgments Direct-injection Engine Research Consortium (DERC) US Department of Energy/Sandia
Maximizing Engine Efficiency by Controlling Fuel Reactivity Using Conventional and Alternative Fuels Sage Kokjohn Acknowledgments Direct-injection Engine Research Consortium (DERC) US Department of Energy/Sandia
1-3 Alkanes structures and Properties :
 1-3 Alkanes structures and Properties : The simplest family of organic molecules is the (Alkanes). Alkanes are relatively unreactive and not often involved in chemical reactions, but they nevertheless
1-3 Alkanes structures and Properties : The simplest family of organic molecules is the (Alkanes). Alkanes are relatively unreactive and not often involved in chemical reactions, but they nevertheless
University of Michigan
 On the Chemical Kinetics of an Unsaturated C7 Ester: Methyl 3 Hexenoate Ignition and Speciation Studies Doctoral Pre-Candidate, Mechanical Engineering Darshan M. A. Karwat Doctoral Candidate, Aerospace
On the Chemical Kinetics of an Unsaturated C7 Ester: Methyl 3 Hexenoate Ignition and Speciation Studies Doctoral Pre-Candidate, Mechanical Engineering Darshan M. A. Karwat Doctoral Candidate, Aerospace
Modeling Constant Volume Chamber Combustion at Diesel Engine Condition
 Modeling Constant Volume Chamber Combustion at Diesel Engine Condition Z. Hu, R.Cracknell*, L.M.T. Somers Combustion Technology Department of Mechanical Engineering Eindhoven University of Technology *Shell
Modeling Constant Volume Chamber Combustion at Diesel Engine Condition Z. Hu, R.Cracknell*, L.M.T. Somers Combustion Technology Department of Mechanical Engineering Eindhoven University of Technology *Shell
Recent Advances in DI-Diesel Combustion Modeling in AVL FIRE A Validation Study
 International Multidimensional Engine Modeling User s Group Meeting at the SAE Congress April 15, 2007 Detroit, MI Recent Advances in DI-Diesel Combustion Modeling in AVL FIRE A Validation Study R. Tatschl,
International Multidimensional Engine Modeling User s Group Meeting at the SAE Congress April 15, 2007 Detroit, MI Recent Advances in DI-Diesel Combustion Modeling in AVL FIRE A Validation Study R. Tatschl,
Eco-diesel engine fuelled with rapeseed oil methyl ester and ethanol. Part 3: combustion processes
 Eco-diesel engine fuelled with rapeseed oil methyl ester and ethanol. Part 3: combustion processes A Kowalewicz Technical University of Radom, al. Chrobrego 45, Radom, 26-600, Poland. email: andrzej.kowalewicz@pr.radom.pl
Eco-diesel engine fuelled with rapeseed oil methyl ester and ethanol. Part 3: combustion processes A Kowalewicz Technical University of Radom, al. Chrobrego 45, Radom, 26-600, Poland. email: andrzej.kowalewicz@pr.radom.pl
Developments in Computational Fluid Dynamics Modeling of Gasoline Direct Injection Engine Combustion and Soot Emission with Chemical Kinetic Modeling
 Developments in Computational Fluid Dynamics Modeling of Gasoline Direct Injection Engine Combustion and Soot Emission with Chemical Kinetic Modeling Abstract Designed to inject gasoline fuel directly
Developments in Computational Fluid Dynamics Modeling of Gasoline Direct Injection Engine Combustion and Soot Emission with Chemical Kinetic Modeling Abstract Designed to inject gasoline fuel directly
STSM Report. Details of the STSM:
 STSM Report Details of the STSM: Visiting researcher: - Name: Luc-Sy Tran - Position: Postdoctoral fellow - Email: luc-sy.tran@uni-bielefeld.de - Tel: +495211062199 - Institute address: Physical Chemistry
STSM Report Details of the STSM: Visiting researcher: - Name: Luc-Sy Tran - Position: Postdoctoral fellow - Email: luc-sy.tran@uni-bielefeld.de - Tel: +495211062199 - Institute address: Physical Chemistry
INFLUENCE OF DIESEL SURROGATES ON THE BEHAVIOR OF SIMPLIFIED SPRAY MODELS
 F2012-A02-012 INFLUENCE OF DIESEL SURROGATES ON THE BEHAVIOR OF SIMPLIFIED SPRAY MODELS 1 Galle, Jonas * ; 1 Verhelst Sebastian 1 Department of Flow, Heat & Combustion Mechanics, Ghent University, Belgium
F2012-A02-012 INFLUENCE OF DIESEL SURROGATES ON THE BEHAVIOR OF SIMPLIFIED SPRAY MODELS 1 Galle, Jonas * ; 1 Verhelst Sebastian 1 Department of Flow, Heat & Combustion Mechanics, Ghent University, Belgium
Revisit of Diesel Reference Fuel (n-heptane) Mechanism Applied to Multidimensional Diesel Ignition and Combustion Simulations
 Seventeenth International Multidimensional Engine Modeling User's Group Meeting at the SAE Congress, April,, Detroit, Michigan Revisit of Diesel Reference Fuel (n-heptane) Mechanism Applied to Multidimensional
Seventeenth International Multidimensional Engine Modeling User's Group Meeting at the SAE Congress, April,, Detroit, Michigan Revisit of Diesel Reference Fuel (n-heptane) Mechanism Applied to Multidimensional
Crankcase scavenging.
 Software for engine simulation and optimization www.diesel-rk.bmstu.ru The full cycle thermodynamic engine simulation software DIESEL-RK is designed for simulating and optimizing working processes of two-
Software for engine simulation and optimization www.diesel-rk.bmstu.ru The full cycle thermodynamic engine simulation software DIESEL-RK is designed for simulating and optimizing working processes of two-
Control of PCCI Combustion using Physical and Chemical Characteristics of Mixed Fuel
 Doshisha Univ. - Energy Conversion Research Center International Seminar on Recent Trend of Fuel Research for Next-Generation Clean Engines December 5th, 27 Control of PCCI Combustion using Physical and
Doshisha Univ. - Energy Conversion Research Center International Seminar on Recent Trend of Fuel Research for Next-Generation Clean Engines December 5th, 27 Control of PCCI Combustion using Physical and
Ignition Delay Measurements of Iso-octane/Ethanol Blend Fuel in a Rapid Compression Machine
 Ignition Delay Measurements of Iso-octane/Ethanol Blend Fuel in a Rapid Compression Machine H. Song, H. H. Song, 1 1 Department of Mechanical and Aerospace Engineering, Seoul National University, Seoul,
Ignition Delay Measurements of Iso-octane/Ethanol Blend Fuel in a Rapid Compression Machine H. Song, H. H. Song, 1 1 Department of Mechanical and Aerospace Engineering, Seoul National University, Seoul,
CONTROLLING COMBUSTION IN HCCI DIESEL ENGINES
 CONTROLLING COMBUSTION IN HCCI DIESEL ENGINES Nicolae Ispas *, Mircea Năstăsoiu, Mihai Dogariu Transilvania University of Brasov KEYWORDS HCCI, Diesel Engine, controlling, air-fuel mixing combustion ABSTRACT
CONTROLLING COMBUSTION IN HCCI DIESEL ENGINES Nicolae Ispas *, Mircea Năstăsoiu, Mihai Dogariu Transilvania University of Brasov KEYWORDS HCCI, Diesel Engine, controlling, air-fuel mixing combustion ABSTRACT
NOx EMISSIONS OF A MILD COMBUSTION BURNER OPERATED WITH JET FUEL SURROGATES
 NOx EMISSIONS OF A MILD COMBUSTION BURNER OPERATED WITH JET FUEL SURROGATES M. Derudi, R. Rota marco.derudi@polimi.it Politecnico di Milano, Dip. di Chimica, Materiali e Ingegneria Chimica G. Natta / CIIRCO,
NOx EMISSIONS OF A MILD COMBUSTION BURNER OPERATED WITH JET FUEL SURROGATES M. Derudi, R. Rota marco.derudi@polimi.it Politecnico di Milano, Dip. di Chimica, Materiali e Ingegneria Chimica G. Natta / CIIRCO,
The combustion behavior of diesel/cng mixtures in a constant volume combustion chamber
 IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS The combustion behavior of diesel/cng mixtures in a constant volume combustion chamber To cite this article: Firmansyah et al
IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS The combustion behavior of diesel/cng mixtures in a constant volume combustion chamber To cite this article: Firmansyah et al
* Corresponding author
 Characterization of Dual-Fuel PCCI Combustion in a Light-Duty Engine S. L. Kokjohn * and R. D. Reitz Department of Mechanical Engineering University of Wisconsin - Madison Madison, WI 5376 USA Abstract.
Characterization of Dual-Fuel PCCI Combustion in a Light-Duty Engine S. L. Kokjohn * and R. D. Reitz Department of Mechanical Engineering University of Wisconsin - Madison Madison, WI 5376 USA Abstract.
Module7:Advanced Combustion Systems and Alternative Powerplants Lecture 32:Stratified Charge Engines
 ADVANCED COMBUSTION SYSTEMS AND ALTERNATIVE POWERPLANTS The Lecture Contains: DIRECT INJECTION STRATIFIED CHARGE (DISC) ENGINES Historical Overview Potential Advantages of DISC Engines DISC Engine Combustion
ADVANCED COMBUSTION SYSTEMS AND ALTERNATIVE POWERPLANTS The Lecture Contains: DIRECT INJECTION STRATIFIED CHARGE (DISC) ENGINES Historical Overview Potential Advantages of DISC Engines DISC Engine Combustion
Influence of ANSYS FLUENT on Gas Engine Modeling
 Influence of ANSYS FLUENT on Gas Engine Modeling George Martinas, Ovidiu Sorin Cupsa 1, Nicolae Buzbuchi, Andreea Arsenie 2 1 CERONAV 2 Constanta Maritime University Romania georgemartinas@ceronav.ro,
Influence of ANSYS FLUENT on Gas Engine Modeling George Martinas, Ovidiu Sorin Cupsa 1, Nicolae Buzbuchi, Andreea Arsenie 2 1 CERONAV 2 Constanta Maritime University Romania georgemartinas@ceronav.ro,
Finite Element Analysis on Thermal Effect of the Vehicle Engine
 Proceedings of MUCEET2009 Malaysian Technical Universities Conference on Engineering and Technology June 20~22, 2009, MS Garden, Kuantan, Pahang, Malaysia Finite Element Analysis on Thermal Effect of the
Proceedings of MUCEET2009 Malaysian Technical Universities Conference on Engineering and Technology June 20~22, 2009, MS Garden, Kuantan, Pahang, Malaysia Finite Element Analysis on Thermal Effect of the
CHAPTER 8 EFFECTS OF COMBUSTION CHAMBER GEOMETRIES
 112 CHAPTER 8 EFFECTS OF COMBUSTION CHAMBER GEOMETRIES 8.1 INTRODUCTION Energy conservation and emissions have become of increasing concern over the past few decades. More stringent emission laws along
112 CHAPTER 8 EFFECTS OF COMBUSTION CHAMBER GEOMETRIES 8.1 INTRODUCTION Energy conservation and emissions have become of increasing concern over the past few decades. More stringent emission laws along
A Comparison of Numerical Results for an Optically Accessible HSDI Diesel Engine with Experimental Data
 A Comparison of Numerical Results for an Optically Accessible HSDI Diesel Engine with Experimental Data Way Lee Cheng, Robert Wang, Jared Zhao and Chia-fon F. Lee Department of Mechanical and Industrial
A Comparison of Numerical Results for an Optically Accessible HSDI Diesel Engine with Experimental Data Way Lee Cheng, Robert Wang, Jared Zhao and Chia-fon F. Lee Department of Mechanical and Industrial
Surrogate Fuels for Transportation Fuels
 Surrogate Fuels for Transportation Fuels Charles Westbrook Lawrence Livermore National Laboratory December 5, 2007 SEDP Meeting Washington, DC The fuel situation in 1922 looks pretty familiar Thomas Midgley,
Surrogate Fuels for Transportation Fuels Charles Westbrook Lawrence Livermore National Laboratory December 5, 2007 SEDP Meeting Washington, DC The fuel situation in 1922 looks pretty familiar Thomas Midgley,
INVESTIGATION ON EFFECT OF EQUIVALENCE RATIO AND ENGINE SPEED ON HOMOGENEOUS CHARGE COMPRESSION IGNITION COMBUSTION USING CHEMISTRY BASED CFD CODE
 Ghafouri, J., et al.: Investigation on Effect of Equivalence Ratio and Engine Speed on... THERMAL SCIENCE: Year 2014, Vol. 18, No. 1, pp. 89-96 89 INVESTIGATION ON EFFECT OF EQUIVALENCE RATIO AND ENGINE
Ghafouri, J., et al.: Investigation on Effect of Equivalence Ratio and Engine Speed on... THERMAL SCIENCE: Year 2014, Vol. 18, No. 1, pp. 89-96 89 INVESTIGATION ON EFFECT OF EQUIVALENCE RATIO AND ENGINE
A view on the functioning mechanism of EBW detonators-part 3: explosive initiation characterisation
 Journal of Physics: Conference Series OPEN ACCESS A view on the functioning mechanism of EBW detonators-part 3: explosive initiation characterisation To cite this article: E A Lee et al 2014 J. Phys.:
Journal of Physics: Conference Series OPEN ACCESS A view on the functioning mechanism of EBW detonators-part 3: explosive initiation characterisation To cite this article: E A Lee et al 2014 J. Phys.:
CHAPTER 1 INTRODUCTION
 1 CHAPTER 1 INTRODUCTION 1.1 GENERAL Diesel engines are the primary power source of vehicles used in heavy duty applications. The heavy duty engine includes buses, large trucks, and off-highway construction
1 CHAPTER 1 INTRODUCTION 1.1 GENERAL Diesel engines are the primary power source of vehicles used in heavy duty applications. The heavy duty engine includes buses, large trucks, and off-highway construction
is the crank angle between the initial spark and the time when about 10% of the charge is burned. θ θ
 ME 410 Day 30 Phases of Combustion 1. Ignition 2. Early flame development θd θ 3. Flame propagation b 4. Flame termination The flame development angle θd is the crank angle between the initial spark and
ME 410 Day 30 Phases of Combustion 1. Ignition 2. Early flame development θd θ 3. Flame propagation b 4. Flame termination The flame development angle θd is the crank angle between the initial spark and
The influence of thermal regime on gasoline direct injection engine performance and emissions
 IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS The influence of thermal regime on gasoline direct injection engine performance and emissions To cite this article: C I Leahu
IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS The influence of thermal regime on gasoline direct injection engine performance and emissions To cite this article: C I Leahu
A RCM study on DME-methane-mixtures under stoichiometric to fuel-rich conditions
 25 th ICDERS August 2 7, 2015 Leeds, UK A RCM study on DME-methane-mixtures under stoichiometric to fuel-rich conditions Marc Werler, Robert Schießl, Ulrich Maas Karlsruhe Institute of Technology, Institute
25 th ICDERS August 2 7, 2015 Leeds, UK A RCM study on DME-methane-mixtures under stoichiometric to fuel-rich conditions Marc Werler, Robert Schießl, Ulrich Maas Karlsruhe Institute of Technology, Institute
CRN Application to Predict the NOx Emissions for Industrial Combustion Chamber
 CRN Application to Predict the NOx Emissions for Industrial Combustion Chamber Nguyen Thanh Hao 1 & Park Jungkyu 2 1 Heat and Refrigeration Faculty, Industrial University of HoChiMinh City, HoChiMinh,
CRN Application to Predict the NOx Emissions for Industrial Combustion Chamber Nguyen Thanh Hao 1 & Park Jungkyu 2 1 Heat and Refrigeration Faculty, Industrial University of HoChiMinh City, HoChiMinh,
Emissions Characterization for D-EGR Vehicle
 Emissions Characterization for D-EGR Vehicle Cary Henry Advance Science. Applied Technology Baseline GDI Vehicle 2012 Buick Regal GS Buick Regal GS uses state-of-the-art turbocharged, direct-injected gasoline
Emissions Characterization for D-EGR Vehicle Cary Henry Advance Science. Applied Technology Baseline GDI Vehicle 2012 Buick Regal GS Buick Regal GS uses state-of-the-art turbocharged, direct-injected gasoline
Natural Gas fuel for Internal Combustion Engine
 Natural Gas fuel for Internal Combustion Engine L. Bartolucci, S. Cordiner, V. Mulone, V. Rocco University of Rome Tor Vergata Department of Industrial Engineering Outline Introduction Motivations and
Natural Gas fuel for Internal Combustion Engine L. Bartolucci, S. Cordiner, V. Mulone, V. Rocco University of Rome Tor Vergata Department of Industrial Engineering Outline Introduction Motivations and
Train turn restrictions and line plan performance
 Downloaded from orbit.dtu.dk on: Jan 05, 2019 Train turn restrictions and line plan performance Burggraeve, Sofie ; Bull, Simon Henry; Lusby, Richard Martin ; Vansteenwegen, Pieter Publication date: 2016
Downloaded from orbit.dtu.dk on: Jan 05, 2019 Train turn restrictions and line plan performance Burggraeve, Sofie ; Bull, Simon Henry; Lusby, Richard Martin ; Vansteenwegen, Pieter Publication date: 2016
Generation of Comprehensive Surrogate Kinetic Models and Validation Databases for Simulating Large Molecular Weight Hydrocarbon Fuels
 Generation of Comprehensive Surrogate Kinetic Models and Validation Databases for Simulating Large Molecular Weight Hydrocarbon Fuels Principal Investigator: Prof. Frederick L. Dryer Other Co-Investigators
Generation of Comprehensive Surrogate Kinetic Models and Validation Databases for Simulating Large Molecular Weight Hydrocarbon Fuels Principal Investigator: Prof. Frederick L. Dryer Other Co-Investigators
POSIBILITIES TO IMPROVED HOMOGENEOUS CHARGE IN INTERNAL COMBUSTION ENGINES, USING C.F.D. PROGRAM
 POSIBILITIES TO IMPROVED HOMOGENEOUS CHARGE IN INTERNAL COMBUSTION ENGINES, USING C.F.D. PROGRAM Alexandru-Bogdan Muntean *, Anghel,Chiru, Ruxandra-Cristina (Dica) Stanescu, Cristian Soimaru Transilvania
POSIBILITIES TO IMPROVED HOMOGENEOUS CHARGE IN INTERNAL COMBUSTION ENGINES, USING C.F.D. PROGRAM Alexandru-Bogdan Muntean *, Anghel,Chiru, Ruxandra-Cristina (Dica) Stanescu, Cristian Soimaru Transilvania
Overview & Perspectives for Internal Combustion Engine using STAR-CD. Marc ZELLAT
 Overview & Perspectives for Internal Combustion Engine using STAR-CD Marc ZELLAT TOPICS Quick overview of ECFM family models Examples of validation for Diesel and SI-GDI engines Introduction to multi-component
Overview & Perspectives for Internal Combustion Engine using STAR-CD Marc ZELLAT TOPICS Quick overview of ECFM family models Examples of validation for Diesel and SI-GDI engines Introduction to multi-component
Influence of Fuel Injector Position of Port-fuel Injection Retrofit-kit to the Performances of Small Gasoline Engine
 Influence of Fuel Injector Position of Port-fuel Injection Retrofit-kit to the Performances of Small Gasoline Engine M. F. Hushim a,*, A. J. Alimin a, L. A. Rashid a and M. F. Chamari a a Automotive Research
Influence of Fuel Injector Position of Port-fuel Injection Retrofit-kit to the Performances of Small Gasoline Engine M. F. Hushim a,*, A. J. Alimin a, L. A. Rashid a and M. F. Chamari a a Automotive Research
Zürich Testing on Fuel Effects and Future Work Programme
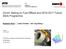 Zürich Testing on Fuel Effects and 2016-2017 Future Work Programme Benjamin Brem 1,2, Lukas Durdina 1,2 and Jing Wang 1,2 1 Empa 2 ETH Zürich FORUM on Aviation and Emissions Workshop Amsterdam 15.04.2016
Zürich Testing on Fuel Effects and 2016-2017 Future Work Programme Benjamin Brem 1,2, Lukas Durdina 1,2 and Jing Wang 1,2 1 Empa 2 ETH Zürich FORUM on Aviation and Emissions Workshop Amsterdam 15.04.2016
Application of Detailed Soot-Particle Model to Simulations of Fundamental Spray Experiments and GDI Engine
 International Multidimensional Engine Modeling User's Group Meeting April 7, 2014, Detroit, Michigan Application of Detailed Soot-Particle Model to Simulations of Fundamental Spray Experiments and GDI
International Multidimensional Engine Modeling User's Group Meeting April 7, 2014, Detroit, Michigan Application of Detailed Soot-Particle Model to Simulations of Fundamental Spray Experiments and GDI
A PFA method for the reduction of Iso-octane
 A PFA method for the reduction of Iso-octane Naman Sharma Department of Mechanical Engineering, Colorado State University, USA Email: nmnsharma27@gmail.com Abstract---- The depleting fossil fuel is raising
A PFA method for the reduction of Iso-octane Naman Sharma Department of Mechanical Engineering, Colorado State University, USA Email: nmnsharma27@gmail.com Abstract---- The depleting fossil fuel is raising
Ignition Strategies for Fuel Mixtures in Catalytic Microburners.
 Ignition Strategies for Fuel Mixtures in Catalytic Microburners. V I K R A M S E S H A D R I AND N I K E T S. K A I S A R C O M B U S T I O N T H E O RY AND M O D E L L I N G VOL. 1 4, N O. 1, 2 0 1 0,
Ignition Strategies for Fuel Mixtures in Catalytic Microburners. V I K R A M S E S H A D R I AND N I K E T S. K A I S A R C O M B U S T I O N T H E O RY AND M O D E L L I N G VOL. 1 4, N O. 1, 2 0 1 0,
I. Ježek et al. Correspondence to: I. Ježek and G. Močnik
 Supplement of Atmos. Chem. Phys. Discuss., 1, 1 1, 01 http://www.atmos-chem-phys-discuss.net/1/1/01/ doi:.1/acpd-1-1-01-supplement Author(s) 01. CC Attribution.0 License. Supplement of Black carbon, particle
Supplement of Atmos. Chem. Phys. Discuss., 1, 1 1, 01 http://www.atmos-chem-phys-discuss.net/1/1/01/ doi:.1/acpd-1-1-01-supplement Author(s) 01. CC Attribution.0 License. Supplement of Black carbon, particle
THE 3-D SIMULATION WITH DETAILED CHEMICAL KINETICS OF THE TURBULENT COMBUSTION IN A PRE-CHAMBER INDIRECT INJECTION DIESEL ENGINE
 Seventh International Conference on CFD in the Minerals and Process Industries CSIRO, Melbourne, Australia 9-11 December 9 THE 3-D SIMULATION WITH DETAILED CHEMICAL KINETICS OF THE TURBULENT COMBUSTION
Seventh International Conference on CFD in the Minerals and Process Industries CSIRO, Melbourne, Australia 9-11 December 9 THE 3-D SIMULATION WITH DETAILED CHEMICAL KINETICS OF THE TURBULENT COMBUSTION
CRN Application to Predict the NOx Emissions for Industrial Combustion Chamber
 Asian Journal of Applied Science and Engineering, Volume 2, No 2/2013 ISSN 2305-915X(p); 2307-9584(e) CRN Application to Predict the NOx Emissions for Industrial Combustion Chamber Nguyen Thanh Hao 1,
Asian Journal of Applied Science and Engineering, Volume 2, No 2/2013 ISSN 2305-915X(p); 2307-9584(e) CRN Application to Predict the NOx Emissions for Industrial Combustion Chamber Nguyen Thanh Hao 1,
THERMO-KINETIC COMBUSTION MODELING OF AN HCCI ENGINE TO ANALYZE IGNITION TIMING FOR CONTROL APPLICATIONS
 THERMO-KINETIC COMBUSTION MODELING OF AN HCCI ENGINE TO ANALYZE IGNITION TIMING FOR CONTROL APPLICATIONS M. SHAHBAKHTI, C. R. KOCH Mechanical Engineering Department, University of Alberta, Canada ABSTRACT
THERMO-KINETIC COMBUSTION MODELING OF AN HCCI ENGINE TO ANALYZE IGNITION TIMING FOR CONTROL APPLICATIONS M. SHAHBAKHTI, C. R. KOCH Mechanical Engineering Department, University of Alberta, Canada ABSTRACT
Modelling Combustion in DI-SI using the G-equation Method and Detailed Chemistry: Emissions and knock. M.Zellat, D.Abouri, Y.Liang, C.
 Modelling Combustion in DI-SI using the G-equation Method and Detailed Chemistry: Emissions and knock Realize innovation. M.Zellat, D.Abouri, Y.Liang, C.Kralj Main topics of the presentation 1. Context
Modelling Combustion in DI-SI using the G-equation Method and Detailed Chemistry: Emissions and knock Realize innovation. M.Zellat, D.Abouri, Y.Liang, C.Kralj Main topics of the presentation 1. Context
Effect of Reformer Gas on HCCI Combustion- Part II: Low Octane Fuels
 Effect of Reformer Gas on HCCI Combustion- Part II: Low Octane Fuels Vahid Hosseini, and M David Checkel Mechanical Engineering University of Alberta, Edmonton, Canada project supported by Auto21 National
Effect of Reformer Gas on HCCI Combustion- Part II: Low Octane Fuels Vahid Hosseini, and M David Checkel Mechanical Engineering University of Alberta, Edmonton, Canada project supported by Auto21 National
Numerical Study of Flame Lift-off and Soot Formation in Diesel Fuel Jets
 Numerical Study of Flame Lift-off and Soot Formation in Diesel Fuel Jets Song-Charng Kong*, Yong Sun and Rolf D. Reitz Engine Research Center, Department of Mechanical Engineering University of Wisconsin
Numerical Study of Flame Lift-off and Soot Formation in Diesel Fuel Jets Song-Charng Kong*, Yong Sun and Rolf D. Reitz Engine Research Center, Department of Mechanical Engineering University of Wisconsin
AN EXPERIMENT STUDY OF HOMOGENEOUS CHARGE COMPRESSION IGNITION COMBUSTION AND EMISSION IN A GASOLINE ENGINE
 THERMAL SCIENCE: Year 2014, Vol. 18, No. 1, pp. 295-306 295 AN EXPERIMENT STUDY OF HOMOGENEOUS CHARGE COMPRESSION IGNITION COMBUSTION AND EMISSION IN A GASOLINE ENGINE by Jianyong ZHANG *, Zhongzhao LI,
THERMAL SCIENCE: Year 2014, Vol. 18, No. 1, pp. 295-306 295 AN EXPERIMENT STUDY OF HOMOGENEOUS CHARGE COMPRESSION IGNITION COMBUSTION AND EMISSION IN A GASOLINE ENGINE by Jianyong ZHANG *, Zhongzhao LI,
INFLUENCE OF FUEL TYPE AND INTAKE AIR PROPERTIES ON COMBUSTION CHARACTERISTICS OF HCCI ENGINE
 ENGINEERING FOR RURAL DEVELOPMENT Jelgava, 23.-24.5.213. INFLUENCE OF FUEL TYPE AND INTAKE AIR PROPERTIES ON COMBUSTION CHARACTERISTICS OF HCCI ENGINE Kastytis Laurinaitis, Stasys Slavinskas Aleksandras
ENGINEERING FOR RURAL DEVELOPMENT Jelgava, 23.-24.5.213. INFLUENCE OF FUEL TYPE AND INTAKE AIR PROPERTIES ON COMBUSTION CHARACTERISTICS OF HCCI ENGINE Kastytis Laurinaitis, Stasys Slavinskas Aleksandras
White Paper. Improving Accuracy and Precision in Crude Oil Boiling Point Distribution Analysis. Introduction. Background Information
 Improving Accuracy and Precision in Crude Oil Boiling Point Distribution Analysis. Abstract High Temperature Simulated Distillation (High Temp SIMDIS) is one of the most frequently used techniques to determine
Improving Accuracy and Precision in Crude Oil Boiling Point Distribution Analysis. Abstract High Temperature Simulated Distillation (High Temp SIMDIS) is one of the most frequently used techniques to determine
Ignition delay studies on hydrocarbon fuel with and without additives
 Ignition delay studies on hydrocarbon fuel with and without additives M. Nagaboopathy 1, Gopalkrishna Hegde 1, K.P.J. Reddy 1, C. Vijayanand 2, Mukesh Agarwal 2, D.S.S. Hembram 2, D. Bilehal 2, and E.
Ignition delay studies on hydrocarbon fuel with and without additives M. Nagaboopathy 1, Gopalkrishna Hegde 1, K.P.J. Reddy 1, C. Vijayanand 2, Mukesh Agarwal 2, D.S.S. Hembram 2, D. Bilehal 2, and E.
Evolution of Particle Size Distribution within the Engine Exhaust and Aftertreatment System
 Evolution of Particle Size Distribution within the Engine Exhaust and Aftertreatment System A. J. Smallbone (1, 2), D. Z. Y. Tay (2), W. L. Heng (2), S. Mosbach (2), A. York (2,3), M. Kraft (2) (1) cmcl
Evolution of Particle Size Distribution within the Engine Exhaust and Aftertreatment System A. J. Smallbone (1, 2), D. Z. Y. Tay (2), W. L. Heng (2), S. Mosbach (2), A. York (2,3), M. Kraft (2) (1) cmcl
System Simulation for Aftertreatment. LES for Engines
 System Simulation for Aftertreatment LES for Engines Christopher Rutland Engine Research Center University of Wisconsin-Madison Acknowledgements General Motors Research & Development Caterpillar, Inc.
System Simulation for Aftertreatment LES for Engines Christopher Rutland Engine Research Center University of Wisconsin-Madison Acknowledgements General Motors Research & Development Caterpillar, Inc.
HERCULES-2 Project. Deliverable: D8.8
 HERCULES-2 Project Fuel Flexible, Near Zero Emissions, Adaptive Performance Marine Engine Deliverable: D8.8 Study an alternative urea decomposition and mixer / SCR configuration and / or study in extended
HERCULES-2 Project Fuel Flexible, Near Zero Emissions, Adaptive Performance Marine Engine Deliverable: D8.8 Study an alternative urea decomposition and mixer / SCR configuration and / or study in extended
THE THEORETICAL STUDY ON INFLUENCE OF FUEL INJECTION PRESSURE ON COMBUSTION PARAMETERS OF THE MARINE 4-STROKE ENGINE
 Journal of KONES Powertrain and Transport, Vol. 23, No. 1 2016 THE THEORETICAL STUDY ON INFLUENCE OF FUEL INJECTION PRESSURE ON COMBUSTION PARAMETERS OF THE MARINE 4-STROKE ENGINE Jerzy Kowalski Gdynia
Journal of KONES Powertrain and Transport, Vol. 23, No. 1 2016 THE THEORETICAL STUDY ON INFLUENCE OF FUEL INJECTION PRESSURE ON COMBUSTION PARAMETERS OF THE MARINE 4-STROKE ENGINE Jerzy Kowalski Gdynia
Learning Guide for Chapter 4 - Alkanes
 Learning Guide for Chapter 4 - Alkanes I. Introduction to Alkanes - p 1 II. Physical Properties, sources, uses and spectroscopy of alkanes - p 3 III. Reactions of alkanes - p 5 IV. Nomenclature of alkanes
Learning Guide for Chapter 4 - Alkanes I. Introduction to Alkanes - p 1 II. Physical Properties, sources, uses and spectroscopy of alkanes - p 3 III. Reactions of alkanes - p 5 IV. Nomenclature of alkanes
Internal Combustion Engines
 Thermochemistry & Fuels Lecture 4 1 Outline In this lecture we will discuss the properties and characteristics of diesel fuels: Cetane number and index Viscosity and cold behaviour Flash point Sulphur
Thermochemistry & Fuels Lecture 4 1 Outline In this lecture we will discuss the properties and characteristics of diesel fuels: Cetane number and index Viscosity and cold behaviour Flash point Sulphur
Study on Emission Characteristics Test of Diesel Engine Operating on. Diesel/Methanol Blends
 Study on Emission Characteristics Test of Diesel Engine Operating on Diesel/Methanol Blends Yuanhua Jia1, a, Guifu Wu2,b, Enhui Xing3,c,Ping Hang 4,d,Wanjiang Wu5e 1,2,3, 4,5 College of Mechanical Engineering
Study on Emission Characteristics Test of Diesel Engine Operating on Diesel/Methanol Blends Yuanhua Jia1, a, Guifu Wu2,b, Enhui Xing3,c,Ping Hang 4,d,Wanjiang Wu5e 1,2,3, 4,5 College of Mechanical Engineering
EFFECTS OF INTAKE AIR TEMPERATURE ON HOMOGENOUS CHARGE COMPRESSION IGNITION COMBUSTION AND EMISSIONS WITH GASOLINE AND n-heptane
 THERMAL SCIENCE: Year 2015, Vol. 19, No. 6, pp. 1897-1906 1897 EFFECTS OF INTAKE AIR TEMPERATURE ON HOMOGENOUS CHARGE COMPRESSION IGNITION COMBUSTION AND EMISSIONS WITH GASOLINE AND n-heptane by Jianyong
THERMAL SCIENCE: Year 2015, Vol. 19, No. 6, pp. 1897-1906 1897 EFFECTS OF INTAKE AIR TEMPERATURE ON HOMOGENOUS CHARGE COMPRESSION IGNITION COMBUSTION AND EMISSIONS WITH GASOLINE AND n-heptane by Jianyong
The influence of fuel injection pump malfunctions of a marine 4-stroke Diesel engine on composition of exhaust gases
 Article citation info: LEWIŃSKA, J. The influence of fuel injection pump malfunctions of a marine 4-stroke Diesel engine on composition of exhaust gases. Combustion Engines. 2016, 167(4), 53-57. doi:10.19206/ce-2016-405
Article citation info: LEWIŃSKA, J. The influence of fuel injection pump malfunctions of a marine 4-stroke Diesel engine on composition of exhaust gases. Combustion Engines. 2016, 167(4), 53-57. doi:10.19206/ce-2016-405
3.2 The alkanes. Isomerism: Alkanes with 4 or more carbons show a type of structural isomerism called chain isomerism
 3.2 The alkanes Prior knowledge: Types of formula general, empirical, molecular, structural, displayed and skeletal. Nomenclature Structural isomers chain and position isomers Free radicals Aliphatic Alkanes
3.2 The alkanes Prior knowledge: Types of formula general, empirical, molecular, structural, displayed and skeletal. Nomenclature Structural isomers chain and position isomers Free radicals Aliphatic Alkanes
Effects of Dilution Flow Balance and Double-wall Liner on NOx Emission in Aircraft Gas Turbine Engine Combustors
 Effects of Dilution Flow Balance and Double-wall Liner on NOx Emission in Aircraft Gas Turbine Engine Combustors 9 HIDEKI MORIAI *1 Environmental regulations on aircraft, including NOx emissions, have
Effects of Dilution Flow Balance and Double-wall Liner on NOx Emission in Aircraft Gas Turbine Engine Combustors 9 HIDEKI MORIAI *1 Environmental regulations on aircraft, including NOx emissions, have
SHOCK IGNITION OF N-HEPTANE WITH SUPPLEMENTAL HYDROGEN
 SHOCK IGNITION OF N-HEPTANE WITH SUPPLEMENTAL HYDROGEN by JD MacLean A thesis submitted to the Department of Mechanical and Materials Engineering In conformity with the requirements for the degree of Master
SHOCK IGNITION OF N-HEPTANE WITH SUPPLEMENTAL HYDROGEN by JD MacLean A thesis submitted to the Department of Mechanical and Materials Engineering In conformity with the requirements for the degree of Master
