(12) United States Patent (10) Patent No.: US 8,999,277 B2
|
|
|
- Kristina Ward
- 5 years ago
- Views:
Transcription
1 US B2 (12) United States Patent () Patent No.: Ayyappan et al. (45) Date of Patent: *Apr. 7, 2015 (54) DIESELEXHAUST FLUID FORMULATION (58) Field of Classification Search (71) (72) (73) (*) (21) (22) (65) (63) (51) (52) THAT REDUCESUREADEPOSITSN EXHAUST SYSTEMS Applicant: Deere & Company, Moline, IL (US) Inventors: Ponnaiyan Ayyappan, Cedar Falls, IA (US); Danan Dou, Cedar Falls, IA (US); Thomas Miller Harris, Waterloo, IA (US) Assignee: Deere & Company, Moline, IL (US) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 U.S.C. 154(b) by 0 days. This patent is Subject to a terminal dis claimer. Appl. No.: 14/155,925 Filed: Jan. 15, 2014 Prior Publication Data US 2014/O A1 Dec. 18, 2014 Related U.S. Application Data Continuation-in-part of application No. 13/919,924, filed on Jun. 17, Int. C. BOLD 53/92 ( ) BOLD 53/94 ( ) C09K3/00 ( ) FOIN3/08 ( ) FOIN3/ ( ) U.S. C. CPC... BOID 53/9409 ( ) CPC... B01D 53/56; B01D 53/78; B01D 53/9404; B01D 53/9418; C09K3/00; F01N3/206 USPC /212, 213.2; 252/182.12, , 252/1881; 60/295, 299 See application file for complete search history. (56) References Cited U.S. PATENT DOCUMENTS 5,171,554 A 12, 1992 Gardner-Chavis et al. 6,511,644 B1 1/2003 MacArthur et al ,241 B2 * 5/2011 Schmelzle et al , ,133,460 B2 3/2012 Arvola et al. 2013/02O7035 A1 8, 2013 Meesen FOREIGN PATENT DOCUMENTS JP A, 2000 * cited by examiner Primary Examiner Timothy Vanoy (74) Attorney, Agent, or Firm Faegre Baker Daniels LLP (57) ABSTRACT A Diesel Exhaust Fluid (DEF) that includes urea, demineral ized water and between 5 and 300 ppm formaldehyde, acetal dehyde, propianaldehyde, or butyraldehyde, and this formu lation of DEF include less than 0.6 ppm of phosphates, calcium, iron, aluminum, magnesium, Sodium, and potas sium, the formulation also includes less than 0.3 ppm copper, Zinc, chromium, and nickel. This formulation of DEF reduces the accumulation of urea deposit in the diesel exhaust system relative to other formulation of specification grade DEF that include less formaldehyde. 21 Claims, 4 Drawing Sheets
2 U.S. Patent Apr. 7, 2015 Sheet 1 of 4 woxo f s {-1/ aaaaaa xx s
3 U.S. Patent Apr. 7, 2015 Sheet 2 of 4 unit sier A sixer A wn-itser B & river 3 ar, S. "r & ics s., Frnsidehyde wit: FG. 2
4 U.S. Patent Apr. 7, 2015 Sheet 3 of 4 N 8 112? He FIG. 3
5 U.S. Patent Apr. 7, 2015 Sheet 4 of 4
6 1. DESELEXHAUST FLUID FORMULATION THAT REDUCESUREADEPOSTSN EXHAUST SYSTEMS CROSS REFERENCE TO RELATED APPLICATIONS This application is a Continuation-in-Part Application of U.S. patent application Ser. No. 13/919,924 filed Jun. 17, 2013, which has been allowed, the complete disclosure of which is hereby expressly incorporated by reference. FIELD OF THE INVENTION This invention relates generally to formulations of Diesel Exhaust Fluid ( DEF') that include low levels of formalde hyde, or other aldehydes including but not limited to acetal dehyde, propianaldehyde, or butyraldehyde, and reduce the deposition of urea in the exhaust systems of engines that use DEF requiring Selective Catalytic Reduction ( SCR) cata lysts. BACKGROUND Diesel engines are the preferred means of producing torque for use in a wide range of applications ranging from uses in transportation Such as heavy-duty trucks and trains, off-road agricultural and mining equipment to the large scale produc tion of on-site electrical power to name a few. Their virtually unmatched power to mass ratios and the relative safety of their fuel makes them almost the only choice for use in appli cations such as long-haul trucks, tractors, earth movers, com bines, Surface mining equipment, non-electric locomotives, high capacity emergency power generators and the like. Diesel engines operate at high internal temperature. One consequence of their high operating temperatures is that at least some of the Nitrogen present in the engine at the moment of combustion may combine with Oxygen to form NO, including species such as NO and NO. Another consequence of their high operating temperatures is that diesel exhaust at or near the point of exit from the engine is very hot. A compound such as NO, is problematic because it readily combines with Volatile organic compounds in the atmosphere to form Smog. NO, is regarded as a pollutant and virtually every industrialized nation regulates the levels of NO, that can be legally discharged into the atmosphere. The regulation governing NO emissions are expected to become even more strict. Fortunately, engine and equipment manufacturers have developed systems for reducing the levels of NO produced by the combustion of diesel fuel and released into the envi ronment. Still, with even tighter limits on the amounts of these compounds that can be released into the atmosphere there remains a need for improved materials and methods for reducing the levels of NO; some aspects of the instant inven tion address this need. SUMMARY Some embodiments provide formulations of Diesel Exhaust Fluid ( DEF') that include about 32.5 wt.%urea and at least 0.00 wt.% formaldehyde, and demineral The inventive formulations of DEF include less than 0.6 ppm of phosphates, calcium, iron, aluminium, magnesium, Sodium, and potassium, the formulation also includes less than 0.3 ppm copper, Zinc, chromium, and nickel In some embodiments, the DEF formulation includes about wt.% formaldehyde. Still other embodiment include between about 0.00 wt.% to about 0.3 wt.% formaldehyde. In some embodiments, the formulation comprises between about 15.0 wt.% to about 40.0 wt.% urea; between about 15.0 wt.% to about 40.0 wt.% ammonium carbamate; at least 0.00 wt.% formaldehyde, and between about 40.0 wt.% to about 60.0 wt.% water. Some embodiments include formulations for reducing oxides of nitrogen, comprising: between about 19.0 wt.% to about 30.0 wt.% urea; between about 15.0 wt.% to about 40.0 wt.% ammonium carbamate; at least 0.00 wt.% formal dehyde; and between about 40.0 wt.% to about 60.0 wt.% water. In some embodiments the formulations include: between about 19.0 wt.% to about 25.0 wt.% urea; between about 20.0 wt.% to about 35.0 wt.% ammonium carbamate; at least 0.00 wt.% formaldehyde; and between about 45.0 wt.% to about wt. 55.0% water. And in still other embodi ments the formulations include between about 19.0 wt.% to about 22.0 wt.% urea; between about 30.0 wt.% to about 35.0 wt.% ammonium carbamate; at least 0.00 wt.% formal dehyde; and between about 45.0 wt.% to about 50.0 wt.% Water. Yet other embodiments include methods for reducing an oxide of nitrogen, comprising the steps of providing a for mulation, wherein the formulation includes: urea, ammo nium carbamate and demineralized water and is suitable for use in Selective Catalytic Reduction ( SCR) of NO. In Some embodiments, the formulation comprises between about 15.0 wt.% to about 40.0 wt.%urea; between about 15.0 wt.% to about 40.0 wt.% ammonium carbamate; at least 0.00 wt.% formaldehyde, and between about 40.0 wt.% to about 60.0 wt.% water. Yet other embodiments include adding ammonium car bamate as a readily soluble powder to a tank that already includes standard DEF. In some embodiments, these methods include the step of Supplying pre-packaged quantities of pow dered ammonium carbamate which can be added to a reduc tant tank that includes urea. In some embodiments, these methods include the step of determining the composition of the reductant to insure that the relative levels of water, urea and ammonium carbamate in the reductant system are Suit able for use in SCR and the new mixtures exhibit lower freezing temperatures than conventional DEF. In some embodiments, the methods for reducing oxides of nitrogen include the steps of Supplying at least one SCR catalyst and contacting the SCR catalyst with said formula tion. Some embodiments include the step of measuring the composition of said formulation. While still other embodi ments include the further step of adding a portion of ammo nium carbamate to a formulation of reductants. Some embodiments provide DEF formulations suitable for use in methods and/or systems that reduce NO, exhaust emis sions that include between about 15.0 wt.% to about 40.0 wt. % urea; between about 15.0 wt.% to about 40.0 wt.% ammo nium carbamate; between about 0.00 wt.% to about 0.3 wt. % formaldehyde; and between about 40.0 wt.% to about 60.0 wt.% water. Yet other embodiments include systems for reducing an oxide of nitrogen in an engine exhaust, comprising the steps of a formulation that includes a reductant wherein the for mulation includes: between about 19.0 wt.% to about 30.0 wt.% urea; between about 15.0 wt.% to about 40.0 wt.% ammonium carbamate; between about 0.00 wt.% to about 0.01 wt.% formaldehyde; and between about 40.0 wt.% to
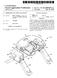
7 3 about 60.0 wt.% water; a SCR catalysts, where the catalyst catalyses the reduction of NO, by said reductant to form products that include N. In some embodiments, the systems for reducingno, emis sions include: reductant formulations having between about 19.0 wt.% to about 25.0 wt.% urea; between about 20.0 wt. % to about 35.0 wt.% ammonium carbamate; between about 0.00 wt.% to about 0.0 wt.% formaldehyde; and between about 45.0 wt.% to about wt. 55.0% water. In some embodiments, the formulations in the systems include: between about 19.0 wt.% to about 22.0 wt.% urea; between about 30.0 wt.% to about 35.0 wt.% ammonium carbamate; between about 0.00 wt.% to about 0.3 wt.% formalde hyde; and between about 45.0 wt.% to about 50.0 wt.% Water. Some embodiments include methods for making spec grade Diesel Exhaust Fluid that has a final level of formalde hyde in the range of wt.% to about 0.01 wt.%, or wt.% to about 0.01 wt.%, or wt.% to about 0.01 wt.%, or wt.% to about 0.05 wt.%, or wt.% to about 0.01 wt.%, or wt.% to about wt.%, or wt. % to about wt.%, or wt.% to about wt.%, or wt.% to about wt.%, these methods include adding a source of formaldehyde Such as formalin to Diesel Exhaust Formulation such that the amount of formaldehyde added to the DEF is between about 0.06 wt % to about 1.0 wt. %, or 0.06 wt % to about 0.80 wt.%, or 0.06 wt % to about 0.60 wt.%, or 0.06 wt % to about 0.50 wt.%, or about 0.06 wit % to about 0.4 wt.%, or 0.1 wt % to about 1.0 wt.%, or 0.1 wit % to about 0.8 wt.%, or 0.1 wt % to about 0.6 wt.%, or 0.1 wit % to about 0.5 wt.%, or 0.1 wt % to about 0.4 wt.%, or about 0. wt.% to about 0.30 wt.% of the final formulation of DEF. Some embodiments include methods and/or systems for adding formaldehyde or otheraldehydes to urea based diesel exhaust formulation. Methods for adding formaldehyde include bubbling or sparging gaseous formaldehyde in an aqueous preparation of exhaust treatment grade urea. Other methods include adding liquid preparations that include formaldehyde Such as formalin to aqueous solution of exhaust treatment grade urea. These additions can be made in bulk supplies before the diesel exhaust formulation is dis persed to individual pieces of equipment. In still other embodiments, individual pieces of equipment can be outfitted with a formaldehyde reservoir that is connected so as to deliver formaldehyde directly into the equipments onboard DEF storage tank or to be co-administered along with DEF directly into the diesel exhaust system. BRIEF DESCRIPTION OF THE FIGURES FIG. 1. Schematic diagram of a representative SCR exhaust treatment system for a diesel engine. FIG. 2. A graph of grams (g) of urea deposited in diesel exhaust system as function of the wt.% of formaldehyde added to spec DEF. FIG. 3. A schematic showing portions of an exemplary diesel exhaust treatment system. FIG. 4. A graph of grams (g) of urea deposited in diesel exhaust system for various aldehydes used in DEF. DESCRIPTION For the purposes of promoting an understanding of the principles of the novel technology, reference will now be made to the preferred embodiments thereof, and specific lan guage will be used to describe the same. It will nevertheless be understood that no limitation of the scope of the novel technology is thereby intended, such alterations, modifica tions, and further applications of the principles of the novel technology being contemplated as would normally occur to one skilled in the art to which the novel technology relates are within the scope of this disclosure and what it claims. Unless implicitly intended or explicitly stated otherwise, the term, about as used herein refers to a range of values including plus or minus percent of the stated value, for example, about 1.0 encompasses the range of values 0.9 to 1.1. As used herein the term demineralized water refers to water that includes very low levels of minerals in general, and in particular very low levels of sulphur, alkaline metals, earth metals, Vanadium, arsenic, ash, or any other compounds that are known to damage SCR catalysts. Demineralized water for use in the inventive formulations can be made by any method known in the art for reducing the level of contaminants in demineralized water including distillation and reverse osmo S1S. Most industrialized nations set limits on the levels of NO, that can be released into the atmosphere by diesel engines. In the United States, the Environmental Protection Agency (EPA) is the agency of the federal government responsible for regulating diesel engine exhaust emissions. The EPA has proffered new regulations governing the levels of NO, that can be legally discharged into the atmosphere by diesel engines powering off-road equipment. These new regulations are referred to as Final Tier 4. The EPA s Final Tier 4 standards require that diesel engines which are operated off road limit their NO emissions to no more than 0.4 g/kw-h. Currently available technology used to reduce the amount of NO, emission emitted from diesel exhaust fumes includes Selective Catalytic Reduction (SCR). This technology is widely used to reduce NO, emissions from heavy duty diesel engines and takes advantage of the high temperatures found in diesel exhaust fumes. Typical chemical reactions catalyzed by SCR catalysts are the reduction of NO, such as NO or NO to N and H2O. In SCR based exhaust treatment systems, the oxidized forms of Nitrogen are reacted with compounds such as ammonia (NH). Some of the reactions that occur on the surface of the SCR catalyst include the following: Diesel Exhaust Fluid (DEF) is a formulation comprising about 32.5 wt.% urea in demineral DEF is widely used to reduce NO and NO to Nina reaction that takes place on the surface of the SCR catalyst. A typical SCR catalyst comprises a large surface area and includes an inertheat Substrate that is coated with at least one stable catalytic material. Substrates used in Such catalysts include ceramic materials; typical catalytic materials include metals and/or metal oxides Such as copper, iron, Vanadium, and the like. The particular combination of catalytic surface material and Substrate for use in a particular application depends in part on a number of factors such as the composi tion of fuel being combusted including, for example, the amount of sulphur in the fuel, the temperature of the exhaust gases, the levels ofno, reduction desired, the reductant being used, the levels and types of other compounds present in the exhaust fumes and the like. Anhydrous ammonia exists predominately as a gas under ambient conditions while aqueous ammonia is formed by
8 5 contacting ammonia with water. Anhydrous ammonia is dif ficult to handle and dangerous if not properly contained. An ammonia carrier Such as urea is safer and easier to handle, store and transport than anhydrous ammonia. Urea plus water exists in liquid form at Standard temperature and pressure. DEF is an especially useful reductant for use in mobile appli cations since it is easier to handle, store and transport than anhydrous ammonia. Accordingly, DEF is a commonly used reductant in SCR diesel exhaust treatment systems used in mobile applications such as heavy trucks and off-road con struction and agricultural equipment. In a typical SCR based exhaust treatment system, a SCR catalyst is positioned in the exhaust stream of a diesel engine. The catalyst is positioned such that the temperature of the exhaust fumes contacting the Surface of the catalyst is high enoughto sustain the reaction of the NO, in the exhaustfumes with the reductant but not so high that the heat produced by the engine and the chemical reactions that take place in the exhaust stream damages the catalyst. Referring now to FIG. 1, a schematic diagram of a typical heavy duty diesel exhaust treatment system (2). An SCR catalyst (4) is positioned within an exhaust pipe (6). The exhaust pipe has two ends. One end (8) is connected to a source of NO () and the other end (12) is vented to the atmosphere (14). A typical system may also include an option additional pair of catalysts, (16) and (18), these are positioned before (16) and after (18) the SCR catalyst (4). The oxidation catalysts catalyse the oxidation of various compounds in the exhaust stream including organic molecules and un-reacted ammonia. Because the SCR system requires a reductant such as ammonia or urea, the SCR system includes a system for storing, and delivering the reductant to the catalyst. Still refer ring to FIG. 1, reductant storage vessel (20) is connected to a first delivery tube (22). First delivery tube (22) has two ends: the first end the inlet (24) of tube (22) is connected to storage vessel (20) while the second end the outlet (26) of tube (22) is connected to a reductant delivery valve (28) that regulates the flow of the reductant from tube (22) to a second delivery tube (30). Tube (30) also has two ends the first end inlet (32) is connected to the outlet of valve (28) while the second end outlet (34) of second delivery tube (32) is connected to the exhaust pipe (6). The outlet (34) of second delivery tube (30) is connected to exhaust pipe (6) Such that the reductant in second delivery tube (30) is delivered onto or near the surface of SCR catalyst (4) by outlet (34). In some embodiments, the SCR system (2) may include a device for maintaining the temperature of the reductant in storage vessel (20). In some configurations, the first reductant delivery tube (22), the reductant delivery value (28) and/or the second reductant delivery tube (30) may also be equipped with a device (40) to help regulate the temperature of the reductant in the system. In some embodiments of the inven tion, the device for regulating the temperature of the reductant (40) may be selected from the group consisting of insulation, a heating coil or sock; and/or a cooling or warming jacket or Some combination thereof. In some embodiments, the system (2) may further include an optional mixing device (43) Supplied to either periodically or continuously agitate the contents of reductant storage ves sel (20). Vessel (20) may also be equipped with a temperature sensor (44) to measure the temperature of the contents of vessel (20). Vessel (20) may also be equipped with a probe (46) for measuring the nitrogen content of the material stored in vessel (20). In some embodiments, the system may be supplied with a controller (42) which may include inputs from sensors connected to the exhaust and/or SCR systems The controller may also be equipped with a Central Process ing Unit or dedicated logic circuits that regulate the disper sion of reductant to the system as necessary to maintain the release of NO, within acceptable limits. The controller may also be used to monitor the temperature or the reductant delivery system and perhaps to control portions of the system dedicated to maintain the reductant within an acceptable tem perature range. In some embodiments, the same controller is used to regulate the rate and/or frequency of the agitator associated with reductant storage tank one. In some embodi ments, the controller may be used to monitor the level of reductant and/or the composition of the reductant in reductant storage vessel (20). Some exhaust systems include an oxida tion catalyst (18), generally located downstream of the SCR catalyst. Some oxidative catalysts can oxidize ammonia and formaldehyde, thereby preventing the release of these com pounds into the atmosphere. Sensors that can be used to monitor the level of compounds that include ammonia and urea in DEF formulation include, but are not limited, to those disclosed in U.S. Pat. No. 7,722, 813 issued on May 25, 20, which is incorporated herein by reference in its entirety. Some of these sensors operate by measuring the ability of a formulation of DEF to transfer heat and correlating this property with the concentration of urea in the system. In some versions the sensor in the form of a probe is inserted into the DEF formulation. In some embodiments, the system includes a circuit used to Supply a current applied to a heating element positioned in a portion of the probe that is submerged in the DEF in order to produce heat and a temperature sensing device that is also Submerged in the DEF. The amount of current that must be applied to the heating element in order to produce a discernable effect on the tem perature sensor is influenced by the composition of the liquid surrounding the probe tip. The relationship between the levels of current that must be applied to affect a temperature change measured at the probe's temperature sensor can be deter mined as a function of urea content in the DEF. Once the relationship between current and urea content is known for a given probe and a formulation with certain components the relationship can then be used to infer the level of urea in a sample of DEF by measuring the amount of current required to effect a change in temperature. Any method that can be used to determine or at least estimate the composition of DEF in a storage tank or anywhere in a SCR system can be used to practice the invention. Spec grade DEF is widely available for use in SCR based NO, reduction systems. Spec grade DEF includes on the order of about 32.5 wt.% urea and purified water. These formulations are optimized to prolong catalyst life and include extremely low levels of impurities that can cause deposits or poison expensive SCR catalysts. Accordingly, SCR spec grade DEF and the formulations disclosed herein have virtually undetectable levels of sulphur, metals, non combustible fillers, other inert contaminants, compounds whose effects on SCR catalyst life are unknown. This technology is well known and has widespread use in Europe, and its use has been growing in North America. Still Some challenges persist in the use of this technology includ ing the tendency for urea deposits to form in the exhaust system especially between the DEF dosing inlet and upstream of selective catalytic reduction (SCR) catalyst and the rela tively high freezing point of 32.5 wt.% urea in water solu tions. This latter problem has been addressed by certain for mulation nitrogen-based reductants which have lower freezing points than aqueous urea and still function. Some of these formulations are disclosed in U.S. patent application
9 7 Ser. No. 13/193,793 filed on Jul. 29, 20, the disclosure of which is incorporated herein by reference in its entirety. The problem of urea deposition in the DEF feeding system can cause reduced fuel efficiency, particulate filter failure, damage to the SCR catalyst bed, and even engine failure as a significant build-up of urea in the exhaust system may cause excessive back pressure. Some exhaust systems are equipped with pressure sensors, in part to detect the effects of urea deposition. These sensors are part of a monitoring system that enables the diesel operator to detect problematic urea build up and to take appropriate action Such as shutting down the system until the deposits can be physically removed from the system. Still other systematic approaches to addressing the problem of urea build-up is to alter the position of the DEF feed tube and/or time the release of DEF into a portion of the exhaust system immediately up-stream of the SCR catalytic bed in order to minimize the time that urea rich DEF is in contact with the DEF feed system and pre-scr section of the exhaust system. Some aspects of the instant invention address the problem ofurea deposition by introducing Small amount of formalde hyde, or other aldehydes including acetaldehyde, propianal dehyde, and butyraldehyde, into DEF. These formulations exhibit an unexpectedly lower tendency to form urea deposits without an apparent effect of SCR catalytic efficiency. As disclosed herein, the addition of small amount of formalde hyde on the order of 0.03 wt.% to spec grade DEF can reduce the urea deposition. Embodiments of the invention include spec. grade DEF mixed with at least 0.08 wt.% formalde hyde, for use in the catalytic reduction ofno, indiesel engine exhaust. Aspects of the invention include formulations of spec grade DEF that include levels of formaldehyde sufficient to significantly reduce the level of urea deposited in the diesel exhaust treatment system. Many of these formulations, much like currently commercially available spec grade DEF, are substantially free of any compound that can affect SCR per formance and half-life. Still other aspects of the invention include introducing urea and levels of formaldehyde sufficient to reduce the deposition of urea in the diesel exhaust system by 90, 95, or even 98 or greater percent that similar DEF formulation that do not include the levels of formaldehyde disclosed herein. Methods for adding formaldehyde directly to spec grade DEF include any method for the addition of such compounds to aqueous solutions. These methods include bubbling or sparging gaseous formaldehyde into aqueous preparations of spec grade DEF until the level of formaldehyde in the DEF reaches the desired levels. Such mixing can be done when DEF is manufactured, or it may be done sometime later, for example, when DEF is added to a DEF container in the commercial outlet or in the tank or other container that holds spec grade DEF. Still another method for adding formaldehyde to DEF is to mix liquid preparations of formaldehyde directly with spec grade DEF. In some embodiments, the formaldehyde mixed with the spec grade DEF is itself in the mixture of com pounds. One Such preparation of formaldehyde that can be mixed with DEF is formalin, a mixture of formaldehyde, methanol, and water. Still another method for using formaldehyde to reduce urea build-up in diesel exhaust systems is to introduce formalde hyde into the mixer along with spec grade DEF. Some of these methods may make use of formaldehyde's low vapour pres Sure (formaldehyde is a gas at room temp with high vapour pressure up to 5 bar). In some embodiments, a high pressure cartridge that includes formaldehyde is used to deliver the requisite level of formaldehyde into a DEF storage tank located at the commercial outlet where spec grade DEF is sold or into a DEF reservoir positioned on the equipment. One advantage of this approach is that it eliminates or at least reduces the customer's exposure to formaldehyde. Examples Referring now to Table 1. Various formulation of DEF were created and tested to determine if the different formulations had a measurable effect on the tendency of urea to deposit in a diesel exhaust treatment system. The engine run parameters used to generate the data Summarized in Table 1, are pre sented in Table 2. Referring again to Table 1. As a control and to determine a base line for urea deposition in the system used to test various formulation of DEF, commercially available DEF was tested. The DEF tested, included 32.5 wt.% urea; a sample of this material was analyzed and was found to include wt.% aldehyde. The required quantities of spec DEF (commercially avail able) and formalin (mixture of water, formaldehyde and methanol) were added and mixed well in a sealed container with a help of magnetic stirrer. The sealed container was placed in 31 C. water bath prior to being tested. Referring next to FIG. 3, a schematic of an exemplary diesel exhaust system (0) is illustrated. Diesel exhaust sys tem (0) illustratively includes exhaust inlet (2), Diesel Oxidation Catalyst ( DOC) (4), Diesel Particulate Filter ( DPF) (6), doser (8), decomposition tube (1), mixer (112), SCR/SCR-AOC ( ammonia oxidation catalyst ) (114), and exhaust outlet (116). SCR/SCR-AOC (114) may be an SCR or a combination of an SCR and an oxidation catalyst. The amount of urea that may be deposited in the exhaust treatment system is a function of factors including engine operating parameters, ambient condition, the posi tioning and/or configuration of the doser (8), and/or mixer (112) with the decomposition tube (1). Referring now to FIG. 2, the effect of doser and mixer on the rate of urea deposition is illustrated by the differences in the amount of urea deposited as shown by the 2 different curves in the figure. In both arrangements the addition of Small amounts of form aldehyde to the DEF reduced the rate at which urea was deposited in the diesel exhaust treatment system. The decomposition tube in the after-treatment system, where one would expect urea to be deposited, was removed and recorded for clean weight at 250 C. The decomposition tube was then installed back into the system before the urea deposit study was started. Prior to injecting the modified DEF into the decomposition tube, circulating coolant was used to maintain the temperature of the dosing module at about 80 C. The test was carried out with a help of flow bench set-up which mimics exact engine conditions. Desired steady state space velocity and stable temperatures were achieved with help of air flow and natural gas combustion respectively. Under specific flow bench and steady state conditions, the required quantities of modified DEF injected into decompo sition tube for 4 hrs (See Table 2). Once the test was com pleted, the decomposition tube was removed and weighed at 250C. The amount of urea deposited in the tube was calcu lated based on initial and final weight of decomposition tube. The amount of urea in the various formulations and the amount of urea deposited in the decomposition tube is sum marized in Table 1. Other formulations that were made and tested for their effect on urea deposition included: 0.11 wt.% methanol, added to specification grade DEF (spec DEF): 0.32 wt.%
10 9 formic acid added to spec DEF: 0.32 wt.%, 0.16 wt.%, 0.08 wt.%, or 0.03 wt.% formalin added to spec DEF. The addi tion of defined amounts of formalin to DEF resulted in a change in the amount of formaldehyde measured in the vari ous mixtures. formaldehyde, other aldehydes including acetaldehyde, pro pianaldehyde, and butyraldehyde have been shown to reduce urea deposits in diesel exhaust systems. These other alde hydes when tested reducedurea deposits between about 39% and about 55%. TABLE 3 Mixtures of DEF and various aldehydes, Added aldehyde weight percent, and the amount of urea (g) deposited in a test diesel exhaust treatment System. Urea Solution mixture Urea Added Resulting deposit wt % aldehyde wt % adlalehyde wt % (g) Urea deposit reduction% DEF 32.5 O O.OOOS 190 O.O Formaldehyde in DEF 32.5 O.32 O.OOS Acetaldehyde in DEF 32.5 O.33 NA Propianaldehyde in DEF 32.5 O.33 NA Butyraldehyde in DEF 32.5 O.33 NA TABLE 1. 2O While the novel technology has been illustrated and described in detail in the figures and foregoing description, Mixtures of DEF and various additives, level of formaldehyde measured the same is to be considered as illustrative and not restrictive in the various mixtures, the amount of urea (g) deposited in a test h it bei d d th lv th f d diesel exhaust treatment System. 1 C aracter, 1t being understood that on y the preferre 25 embodiments have been shown and described and that all Resulting changes and modifications that come within the spirit of the Solution mixture of Spec Added formaldehyde deposit urea urea reduction deposit by novel 1 technol technology are desired esire to O b be pro tected. CCC. A S Well, 11, While whil DEF and Additive wt % (wt %) (g) (%) the novel technology was illustrated using specific examples, theoretical arguments, accounts, and illustrations, these illus DEF: * (a) 190 O.O 30 Methanol in spec DEF O.11 NA trations and the accompanying discussion should by no Formic acid in spec DEF O32 NA means be interpreted as limiting the technology. All patents, Formaldehyde in spec DEF (a O patent applications, and references to texts, Scientific trea Formaldehyde in spec DEF (a) Formaldehyde in spec DEF O.O8 O.OO* tises, publications, and the like referenced in this application Formaldehyde in spec DEF * are incorporated herein by reference in their entirety. **All mixtures in Table 1 include specification-grade DEF, which comprises about 32.5% a determined by chemical analys We claim: gy, emical analys1s 1. A formulation for reducing oxides of nitrogen in diesel engine exhaust, comprising: Note: formaldehyde was added to spec DEF by measured 40 about 19.0 wt.% to about 40 wt.% urea; amounts of Formalin, a mixture of formaldehyde (35-39%), at least 0.2 added wt.% of an aldehyde selected from the methanol (-15%) and water. group consisting of formaldehyde, acetaldehyde, pro Results reported in the table are those obtained using doser pianaldehyde, and butyraldehyde; and A & mixer A. TABLE 2 Engine Test Conditions used to collect the data summarized in Table 1. St eady demineral The formulation according to claim 1, wherein the alde hyde is formaldehyde. 3. The formulation according to claim 1, including: no more than 0.6 ppm of phosphates calcium, iron, alumi num, magnesium, Sodium and potassium; and Test Condition State 50 no more than 0.3 ppm copper, Zinc, chromium, and nickel. 4. The formulation according to claim 3, comprising: Exhaust flow (kg/hr) 350 between about 0.2 added wt.% and about 0.4 added wt.% Exhaust flow temp (C.) 350 DEF flow (g/sec) O.38 of an aldehyde selected from the group consisting of: Test duration (hr) 4 formaldehyde, acetaldehyde, propianaldehyde, and 55 butyraldehyde. 5. Thef lati ding claim3, further including: Referring now to FIG. 2. Some of the data in Table 1 is TOU81O. acco, 1ng to claim 5, Tu O er presented graphically (see lowerline in the figure). The upper in R. E. ofurs this as a this of A). The curve generated with doser Band mixer B (the upper ormaldehyde in the DEF use in the test ( oser A '' 60 curve) illustrates 1) the effect of doser and mixer choice and position on the rate of urea deposition and 2) that less urea accumulates in the exhaust system with either doser and mixer combination when formaldehyde is added to the DEF. 65 Referring now to FIG. 4. The data presented in Table 3 below are presented graphically in FIG. 4. In addition to between about 15.0 wt.% to about 40.0 wt.% ammonium carbamate 6. The formulation according to claim 5, comprising: between about 19.0 wt.% to about 35.0 wt.% urea; between about 15.0 wt.% to about 40.0 wt.% ammonium carbamate: at least 0.2 added wt.% of an aldehyde selected from the group consisting of formaldehyde, acetaldehyde, pro pianaldehyde, and butyraldehyde; between about 40.0 wt.% to about 60.0 wt.% demineral ized water;
11 11 no more than no more than 0.6 ppm of phosphates, cal cium, iron, aluminum, magnesium, sodium and potas sium; and no more than 0.3 ppm copper, Zinc, chromium, and nickel. 7. The formulation according to claim 6, wherein said formulation comprises: between about 19.0 wt.% to about 35.0 wt.% urea; between about 20.0 wt.% to about 35.0 wt.% ammonium carbamate; between about 0.25 added wt.% to about 0.35 added wt.% of an aldehyde selected from the group consisting of: formaldehyde, acetaldehyde, propianaldehyde, and butyraldehyde; and between about 45.0 wt.% to about wt. 55.0% demineral 8. The formulation according to claim 6, wherein said formulation comprises: between about 19.0 wt.% to about 22.0 wt.% urea; between about 30.0 wt.% to about 35.0 wt.% ammonium carbamate; between about 0.28 added wt.% to about 0.34 added wt.% of an aldehyde selected from the group consisting of: formaldehyde, acetaldehyde, propianaldehyde, and butyraldehyde; and between about 45.0 wt.% to about 50.0 wt.% demineral 9. A method for reducing an oxide of nitrogen in diesel exhaust, comprising the steps of providing a Diesel Exhaust Fluid formulation, wherein the formulation includes: urea; ammonium carbamate; an aldehyde selected from the group consisting of form aldehyde, acetaldehyde, propianaldehyde, and butyraldehyde; demineralized water; no more than about 0.6 ppm of phosphates, calcium, iron, aluminum, magnesium, Sodium, and potassium; and no more than 0.3 ppm copper, Zinc, chromium and nickel, wherein said formulation is suitable for use in the Selective Catalytic Reduction of NO, in diesel engine exhaust gases; and introducing the formulation into diesel exhaust Such that the formulation reacts with NO, on the surface of a SCR.. The method according to claim 9, wherein said formu lation, comprises: between about 15.0 wt.% to about 40.0 wt.% urea; between about 15.0 wt.% to about 40.0 wt.% ammonium carbamate; between about 40.0 wt.% to about 60.0 wt.% demineral ized water, and between about 0.2 added wt.% to about 0.4 added wt % of an aldehyde selected from the group consisting of form aldehyde, acetaldehyde, propianaldehyde, and butyral dehyde. 11. The method according to claim 9, wherein said formu lation, comprises: between about 19.0 wt.% to about 35.0 wt.% urea; between about 15.0 wt.% to about 40.0 wt.% ammonium carbamate; and between about 40.0 wt.% to about 60.0 wt.% demineral The method according to claim 9, wherein said formu lation, comprises: between about 19.0 wt.% to about 22.0 wt.% urea; between about 30.0 wt.% to about 35.0 wt.% ammonium carbamate; and between about 45.0 wt.% to about 50.0 wt.% demineral 13. The method according to claim 9, further including the step of: determining the composition of said formulation. 14. The method according to claim 13, wherein the deter mining step includes measuring the amount of urea in said formulation. 15. The method according to claim 14, wherein the deter mining step includes using a sensor and wherein the sensor is in contact with said formulation. 16. The method according to claim 9, further including the step of: adjusting the composition of said formulation by the addi tion of at least one chemical to said formulation, wherein the at least one chemical is selected from the group consisting of urea, ammonium carbamate, formalde hyde, acetaldehyde, propianaldehyde, butyraldehyde and demineral 17. A system for reducing the level of NO, released into the atmosphere by the combustion of diesel fuel; comprising a formulation, wherein said formulation includes: urea; ammonium carbamate; an aldehyde selected from the group consisting of form aldehyde, acetaldehyde, propianaldehyde, and butyraldehyde; and demineralized water, wherein said formulation is suit able for the Selective Catalytic Reduction of NO, in diesel exhaust gases; and a reservoir for holding at one component of said formula tion. 18. The system according to claim 17, wherein the formu lation, comprises: between about 15.0 wt.% to about 40.0 wt.% urea; between about 15.0 wt.% to about 40.0 wt.% ammonium carbamate; between about 0.2 added wt.% to about 0.4 added wt.% of an aldehyde selected from the group consisting of form aldehyde, acetaldehyde, propianaldehyde, and butyral dehyde; and between about 40.0 wt.% to about 60.0 wt.% demineral 19. The system according to claim 17, wherein said formu lation comprises: between about 19.0 wt.% to about 35.0 wt.% urea; between about 20.0 wt.% to about 35.0 wt.% ammonium carbamate; between about 0.25 added wt.% to about 0.35 added wt.% of an aldehyde selected from the group consisting of: formaldehyde; and between about 45.0 wt.% to about wt. 55.0% demineral
12 The system according to claim 17, wherein said formu lation comprises: between about 19.0 wt.% to about 22.0 wt.% urea; between about 30.0 wt.% to about 35.0 wt.% ammonium carbamate; between about 0.28 added wt.% to about 0.34 added wt.% of an aldehyde selected from the group consisting of: formaldehyde, acetaldehyde, propianaldehyde, and butyraldehyde; and between about 45.0 wt.% to about 50.0 wt.% demineral 21. The system according to claim 18, further including: a device for measuring the composition of said formula tion
13 UNITED STATES PATENT AND TRADEMARK OFFICE CERTIFICATE OF CORRECTION PATENT NO. : 8, B2 Page 1 of 1 APPLICATIONNO. : 14/ DATED : April 7, 2015 INVENTOR(S) : Ponnaiyan Ayyappan et al. It is certified that error appears in the above-identified patent and that said Letters Patent is hereby corrected as shown below: In the Drawings, Figure 4, the word formaldehye should read formaldehyde. In the Claims, In Claim 6, Column 11, Line 1, delete the second occurrence of the phrase no more than. Signed and Sealed this Twenty-seventh Day of October, % 4 Michelle K. Lee Director of the United States Patent and Trademark Office
TEPZZ 55_ZZ9A_T EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION. (51) Int Cl.: B01D 53/94 ( )
 (19) TEPZZ _ZZ9A_T (11) EP 2 1 009 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication:.01.13 Bulletin 13/0 (1) Int Cl.: B01D 3/94 (06.01) (21) Application number: 1217.7 (22) Date of filing:
(19) TEPZZ _ZZ9A_T (11) EP 2 1 009 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication:.01.13 Bulletin 13/0 (1) Int Cl.: B01D 3/94 (06.01) (21) Application number: 1217.7 (22) Date of filing:
(12) United States Patent
 US008998577B2 (12) United States Patent Gustafson et al. (10) Patent No.: US 8,998,577 B2 (45) Date of Patent: Apr. 7, 2015 (54) (75) (73) (*) (21) (22) (65) (51) (52) TURBINE LAST STAGE FLOW PATH Inventors:
US008998577B2 (12) United States Patent Gustafson et al. (10) Patent No.: US 8,998,577 B2 (45) Date of Patent: Apr. 7, 2015 (54) (75) (73) (*) (21) (22) (65) (51) (52) TURBINE LAST STAGE FLOW PATH Inventors:
(12) United States Patent (10) Patent No.: US 8,899,031 B2
 US008899.031B2 (12) United States Patent (10) Patent No.: US 8,899,031 B2 Turnis et al. (45) Date of Patent: Dec. 2, 2014 (54) COLD START VALVE (58) Field of Classification Search CPC... F15B 21/042: F15B
US008899.031B2 (12) United States Patent (10) Patent No.: US 8,899,031 B2 Turnis et al. (45) Date of Patent: Dec. 2, 2014 (54) COLD START VALVE (58) Field of Classification Search CPC... F15B 21/042: F15B
(12) United States Patent
 USO09597628B2 (12) United States Patent Kummerer et al. (10) Patent No.: (45) Date of Patent: Mar. 21, 2017 (54) (71) (72) (73) (*) (21) (22) (65) (60) (51) (52) OPTIMIZATION OF A VAPOR RECOVERY UNIT Applicant:
USO09597628B2 (12) United States Patent Kummerer et al. (10) Patent No.: (45) Date of Patent: Mar. 21, 2017 (54) (71) (72) (73) (*) (21) (22) (65) (60) (51) (52) OPTIMIZATION OF A VAPOR RECOVERY UNIT Applicant:
(12) United States Patent (10) Patent No.: US 8,651,070 B2
 USOO8651070B2 (12) United States Patent (10) Patent No.: US 8,651,070 B2 Lindner et al. (45) Date of Patent: Feb. 18, 2014 (54) METHOD AND APPARATUS TO CONTROL USPC... 123/41.02, 41.08-41.1, 41.44, 198C
USOO8651070B2 (12) United States Patent (10) Patent No.: US 8,651,070 B2 Lindner et al. (45) Date of Patent: Feb. 18, 2014 (54) METHOD AND APPARATUS TO CONTROL USPC... 123/41.02, 41.08-41.1, 41.44, 198C
(12) United States Patent (10) Patent No.: US 9,624,044 B2
 USOO9624044B2 (12) United States Patent (10) Patent No.: US 9,624,044 B2 Wright et al. (45) Date of Patent: Apr. 18, 2017 (54) SHIPPING/STORAGE RACK FOR BUCKETS (56) References Cited (71) Applicant: CWS
USOO9624044B2 (12) United States Patent (10) Patent No.: US 9,624,044 B2 Wright et al. (45) Date of Patent: Apr. 18, 2017 (54) SHIPPING/STORAGE RACK FOR BUCKETS (56) References Cited (71) Applicant: CWS
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 US 20140208759A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0208759 A1 Ekanayake et al. (43) Pub. Date: Jul. 31, 2014 (54) APPARATUS AND METHOD FOR REDUCING Publication
US 20140208759A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0208759 A1 Ekanayake et al. (43) Pub. Date: Jul. 31, 2014 (54) APPARATUS AND METHOD FOR REDUCING Publication
o CSF (12) Patent Application Publication (10) Pub. No.: US 2007/ A1 (19) United States NTAKETHROTLE (43) Pub. Date: Oct.
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0227127 A1 Hornby US 20070227127A1 (43) Pub. Date: Oct. 4, 2007 (54) DIESELEXHAUST DOSING VALVE (75) (73) (21) (22) (60) Inventor:
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0227127 A1 Hornby US 20070227127A1 (43) Pub. Date: Oct. 4, 2007 (54) DIESELEXHAUST DOSING VALVE (75) (73) (21) (22) (60) Inventor:
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 US 2011 01 17420A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0117420 A1 Kim et al. (43) Pub. Date: May 19, 2011 (54) BUS BAR AND BATTERY MODULE INCLUDING THE SAME (52)
US 2011 01 17420A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0117420 A1 Kim et al. (43) Pub. Date: May 19, 2011 (54) BUS BAR AND BATTERY MODULE INCLUDING THE SAME (52)
(12) United States Patent (10) Patent No.: US 6,205,840 B1
 USOO620584OB1 (12) United States Patent (10) Patent No.: US 6,205,840 B1 Thompson (45) Date of Patent: Mar. 27, 2001 (54) TIME CLOCK BREATHALYZER 4,749,553 * 6/1988 Lopez et al.... 73/23.3 X COMBINATION
USOO620584OB1 (12) United States Patent (10) Patent No.: US 6,205,840 B1 Thompson (45) Date of Patent: Mar. 27, 2001 (54) TIME CLOCK BREATHALYZER 4,749,553 * 6/1988 Lopez et al.... 73/23.3 X COMBINATION
ia 451s, 10-y (12) Patent Application Publication (10) Pub. No.: US 2003/ A1 (19) United States Johnson et al. (43) Pub. Date: Feb.
 (19) United States US 2003OO29160A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0029160 A1 Johnson et al. (43) Pub. Date: Feb. 13, 2003 (54) COMBINED CYCLE PULSE DETONATION TURBINE ENGINE
(19) United States US 2003OO29160A1 (12) Patent Application Publication (10) Pub. No.: US 2003/0029160 A1 Johnson et al. (43) Pub. Date: Feb. 13, 2003 (54) COMBINED CYCLE PULSE DETONATION TURBINE ENGINE
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150214458A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0214458 A1 Nandigama et al. (43) Pub. Date: Jul. 30, 2015 (54) THERMOELECTRIC GENERATORSYSTEM (52) U.S. Cl.
(19) United States US 20150214458A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0214458 A1 Nandigama et al. (43) Pub. Date: Jul. 30, 2015 (54) THERMOELECTRIC GENERATORSYSTEM (52) U.S. Cl.
USOO582O2OOA United States Patent (19) 11 Patent Number: 5,820,200 Zubillaga et al. (45) Date of Patent: Oct. 13, 1998
 USOO582O2OOA United States Patent (19) 11 Patent Number: Zubillaga et al. (45) Date of Patent: Oct. 13, 1998 54 RETRACTABLE MOTORCYCLE COVERING 4,171,145 10/1979 Pearson, Sr.... 296/78.1 SYSTEM 5,052,738
USOO582O2OOA United States Patent (19) 11 Patent Number: Zubillaga et al. (45) Date of Patent: Oct. 13, 1998 54 RETRACTABLE MOTORCYCLE COVERING 4,171,145 10/1979 Pearson, Sr.... 296/78.1 SYSTEM 5,052,738
Phillips (45) Date of Patent: Jun. 10, (54) TRIPLE CLUTCH MULTI-SPEED (58) Field of Classification Search
 (12) United States Patent US008747274B2 () Patent No.: Phillips () Date of Patent: Jun., 2014 (54) TRIPLE CLUTCH MULTI-SPEED (58) Field of Classification Search TRANSMISSION USPC... 74/3, 331; 475/207
(12) United States Patent US008747274B2 () Patent No.: Phillips () Date of Patent: Jun., 2014 (54) TRIPLE CLUTCH MULTI-SPEED (58) Field of Classification Search TRANSMISSION USPC... 74/3, 331; 475/207
(12) United States Patent
 USOO7324657B2 (12) United States Patent Kobayashi et al. (10) Patent No.: (45) Date of Patent: US 7,324,657 B2 Jan. 29, 2008 (54) (75) (73) (*) (21) (22) (65) (30) Foreign Application Priority Data Mar.
USOO7324657B2 (12) United States Patent Kobayashi et al. (10) Patent No.: (45) Date of Patent: US 7,324,657 B2 Jan. 29, 2008 (54) (75) (73) (*) (21) (22) (65) (30) Foreign Application Priority Data Mar.
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0226455A1 Al-Anizi et al. US 2011 0226455A1 (43) Pub. Date: Sep. 22, 2011 (54) (75) (73) (21) (22) SLOTTED IMPINGEMENT PLATES
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0226455A1 Al-Anizi et al. US 2011 0226455A1 (43) Pub. Date: Sep. 22, 2011 (54) (75) (73) (21) (22) SLOTTED IMPINGEMENT PLATES
I lllll llllllll
 I lllll llllllll 111 1111111111111111111111111111111111111111111111111111111111 US005325666A United States Patent 1191 [ill Patent Number: 5,325,666 Rutschmann [MI Date of Patent: Jul. 5, 1994 [54] EXHAUST
I lllll llllllll 111 1111111111111111111111111111111111111111111111111111111111 US005325666A United States Patent 1191 [ill Patent Number: 5,325,666 Rutschmann [MI Date of Patent: Jul. 5, 1994 [54] EXHAUST
US 7, B2. Loughrin et al. Jan. 1, (45) Date of Patent: (10) Patent No.: and/or the driven component. (12) United States Patent (54) (75)
 USOO7314416B2 (12) United States Patent Loughrin et al. (10) Patent No.: (45) Date of Patent: US 7,314.416 B2 Jan. 1, 2008 (54) (75) (73) (*) (21) (22) (65) (51) (52) (58) (56) DRIVE SHAFT COUPLNG Inventors:
USOO7314416B2 (12) United States Patent Loughrin et al. (10) Patent No.: (45) Date of Patent: US 7,314.416 B2 Jan. 1, 2008 (54) (75) (73) (*) (21) (22) (65) (51) (52) (58) (56) DRIVE SHAFT COUPLNG Inventors:
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1
 US 20070231628A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0231628 A1 Lyle et al. (43) Pub. Date: Oct. 4, 2007 (54) FUEL CELL SYSTEM VENTILATION Related U.S. Application
US 20070231628A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0231628 A1 Lyle et al. (43) Pub. Date: Oct. 4, 2007 (54) FUEL CELL SYSTEM VENTILATION Related U.S. Application
(12) United States Patent (10) Patent No.: US 6,429,647 B1
 USOO6429647B1 (12) United States Patent (10) Patent No.: US 6,429,647 B1 Nicholson (45) Date of Patent: Aug. 6, 2002 (54) ANGULAR POSITION SENSOR AND 5,444,369 A 8/1995 Luetzow... 324/207.2 METHOD OF MAKING
USOO6429647B1 (12) United States Patent (10) Patent No.: US 6,429,647 B1 Nicholson (45) Date of Patent: Aug. 6, 2002 (54) ANGULAR POSITION SENSOR AND 5,444,369 A 8/1995 Luetzow... 324/207.2 METHOD OF MAKING
(12) United States Patent (10) Patent No.: US 8,215,503 B2. Appel et al. (45) Date of Patent: Jul. 10, 2012
 US008215503B2 (12) United States Patent (10) Patent No.: US 8,215,503 B2 Appel et al. (45) Date of Patent: Jul. 10, 2012 (54) CRANE WITH TELESCOPIC BOOM 3,921,819 A * 1 1/1975 Spain... 212,349 4,394,108
US008215503B2 (12) United States Patent (10) Patent No.: US 8,215,503 B2 Appel et al. (45) Date of Patent: Jul. 10, 2012 (54) CRANE WITH TELESCOPIC BOOM 3,921,819 A * 1 1/1975 Spain... 212,349 4,394,108
Kikuiri et al. (45) Date of Patent: Jun. 3, (54) CAPACITIVE PRESSURE SENSOR 5, A 12, 1996 Ko /53
 (12) United States Patent USOO7382599B2 (10) Patent No.: US 7,382,599 B2 Kikuiri et al. (45) Date of Patent: Jun. 3, 2008 (54) CAPACITIVE PRESSURE SENSOR 5,585.311 A 12, 1996 Ko... 438/53 5,656,781 A *
(12) United States Patent USOO7382599B2 (10) Patent No.: US 7,382,599 B2 Kikuiri et al. (45) Date of Patent: Jun. 3, 2008 (54) CAPACITIVE PRESSURE SENSOR 5,585.311 A 12, 1996 Ko... 438/53 5,656,781 A *
EPA Emission Requirements:
 DEF 101 EPA Emission Requirements: In 2010 the Environmental Protection Agency, OEMs, and many trucking fleets committed themselves to a cleaner environment by reducing NOx emissions. To reduce NOx emissions,
DEF 101 EPA Emission Requirements: In 2010 the Environmental Protection Agency, OEMs, and many trucking fleets committed themselves to a cleaner environment by reducing NOx emissions. To reduce NOx emissions,
(12) United States Patent (10) Patent No.: US 9,168,973 B2
 US009 168973B2 (12) United States Patent (10) Patent No.: US 9,168,973 B2 Offe (45) Date of Patent: Oct. 27, 2015 (54) MOTORCYCLE SUSPENSION SYSTEM (56) References Cited (71) Applicant: Andrew Offe, Wilunga
US009 168973B2 (12) United States Patent (10) Patent No.: US 9,168,973 B2 Offe (45) Date of Patent: Oct. 27, 2015 (54) MOTORCYCLE SUSPENSION SYSTEM (56) References Cited (71) Applicant: Andrew Offe, Wilunga
(12) United States Patent (10) Patent No.: US 6,779,516 B1
 USOO6779516B1 (12) United States Patent (10) Patent No.: Shureb () Date of Patent: Aug. 24, 2004 (54) CLOSED CRANKCASE VENTILATION 4.856,487 A * 8/1989 Furuya... 123/574 SYSTEM WITH FLOW METER FOR 5,003,943
USOO6779516B1 (12) United States Patent (10) Patent No.: Shureb () Date of Patent: Aug. 24, 2004 (54) CLOSED CRANKCASE VENTILATION 4.856,487 A * 8/1989 Furuya... 123/574 SYSTEM WITH FLOW METER FOR 5,003,943
USOO58065OOA United States Patent (19) 11 Patent Number: 5,806,500 Fargo et al. (45) Date of Patent: Sep. 15, 1998
 USOO58065OOA United States Patent (19) 11 Patent Number: 5,806,500 Fargo et al. (45) Date of Patent: Sep. 15, 1998 54 FUEL VAPOR RECOVERY SYSTEM 5,456,238 10/1995 Horiuchi et al.. 5,460,136 10/1995 Yamazaki
USOO58065OOA United States Patent (19) 11 Patent Number: 5,806,500 Fargo et al. (45) Date of Patent: Sep. 15, 1998 54 FUEL VAPOR RECOVERY SYSTEM 5,456,238 10/1995 Horiuchi et al.. 5,460,136 10/1995 Yamazaki
(12) United States Patent (10) Patent No.: US 6,695,581 B2
 USOO6695581B2 (12) United States Patent (10) Patent No.: US 6,695,581 B2 Wass0n et al. (45) Date of Patent: Feb. 24, 2004 (54) COMBINATION FAN-FLYWHEEL-PULLEY JP 59-81.835 2/1984 ASSEMBLY AND METHOD OF
USOO6695581B2 (12) United States Patent (10) Patent No.: US 6,695,581 B2 Wass0n et al. (45) Date of Patent: Feb. 24, 2004 (54) COMBINATION FAN-FLYWHEEL-PULLEY JP 59-81.835 2/1984 ASSEMBLY AND METHOD OF
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 US 20090314114A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0314114A1 Grosberg (43) Pub. Date: Dec. 24, 2009 (54) BACKLASH ELIMINATION MECHANISM (22) Filed: Jun. 15,
US 20090314114A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0314114A1 Grosberg (43) Pub. Date: Dec. 24, 2009 (54) BACKLASH ELIMINATION MECHANISM (22) Filed: Jun. 15,
(12) United States Patent
 (12) United States Patent US009277323B2 (10) Patent No.: L0cke et al. (45) Date of Patent: Mar. 1, 2016 (54) COMPACT AUDIO SPEAKER (56) References Cited (71) Applicant: Apple Inc., Cupertino, CA (US) U.S.
(12) United States Patent US009277323B2 (10) Patent No.: L0cke et al. (45) Date of Patent: Mar. 1, 2016 (54) COMPACT AUDIO SPEAKER (56) References Cited (71) Applicant: Apple Inc., Cupertino, CA (US) U.S.
(12) United States Patent (10) Patent No.: US 7,592,736 B2
 US007592736 B2 (12) United States Patent (10) Patent No.: US 7,592,736 B2 Scott et al. (45) Date of Patent: Sep. 22, 2009 (54) PERMANENT MAGNET ELECTRIC (56) References Cited GENERATOR WITH ROTOR CIRCUMIFERENTIALLY
US007592736 B2 (12) United States Patent (10) Patent No.: US 7,592,736 B2 Scott et al. (45) Date of Patent: Sep. 22, 2009 (54) PERMANENT MAGNET ELECTRIC (56) References Cited GENERATOR WITH ROTOR CIRCUMIFERENTIALLY
(12) United States Patent
 (12) United States Patent USOO7357465B2 (10) Patent No.: US 7,357.465 B2 Young et al. (45) Date of Patent: Apr. 15, 2008 (54) BRAKE PEDAL FEEL SIMULATOR 3,719,123 A 3/1973 Cripe 3,720,447 A * 3/1973 Harned
(12) United States Patent USOO7357465B2 (10) Patent No.: US 7,357.465 B2 Young et al. (45) Date of Patent: Apr. 15, 2008 (54) BRAKE PEDAL FEEL SIMULATOR 3,719,123 A 3/1973 Cripe 3,720,447 A * 3/1973 Harned
(12) United States Patent (10) Patent No.: US 6,643,958 B1
 USOO6643958B1 (12) United States Patent (10) Patent No.: Krejci (45) Date of Patent: Nov. 11, 2003 (54) SNOW THROWING SHOVEL DEVICE 3,435,545. A 4/1969 Anderson... 37/223 3,512,279 A 5/1970 Benson... 37/244
USOO6643958B1 (12) United States Patent (10) Patent No.: Krejci (45) Date of Patent: Nov. 11, 2003 (54) SNOW THROWING SHOVEL DEVICE 3,435,545. A 4/1969 Anderson... 37/223 3,512,279 A 5/1970 Benson... 37/244
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0041841 A1 Huazhao et al. US 20140041841A1 (43) Pub. Date: Feb. 13, 2014 (54) (71) (72) (21) (22) (62) (30) MICRO-CHANNEL HEAT
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0041841 A1 Huazhao et al. US 20140041841A1 (43) Pub. Date: Feb. 13, 2014 (54) (71) (72) (21) (22) (62) (30) MICRO-CHANNEL HEAT
(12) United States Patent (10) Patent No.: US 8, B2
 US0087.08325B2 (12) United States Patent (10) Patent No.: US 8,708.325 B2 Hwang et al. (45) Date of Patent: Apr. 29, 2014 (54) PAPER CLAMPINGAPPARATUS FOR (56) References Cited OFFICE MACHINE (75) Inventors:
US0087.08325B2 (12) United States Patent (10) Patent No.: US 8,708.325 B2 Hwang et al. (45) Date of Patent: Apr. 29, 2014 (54) PAPER CLAMPINGAPPARATUS FOR (56) References Cited OFFICE MACHINE (75) Inventors:
(12) United States Patent
 (12) United States Patent USOO9284.05OB2 (10) Patent No.: US 9.284,050 B2 Bagai (45) Date of Patent: Mar. 15, 2016 (54) AIRFOIL FOR ROTOR BLADE WITH (56) References Cited REDUCED PITCHING MOMENT U.S. PATENT
(12) United States Patent USOO9284.05OB2 (10) Patent No.: US 9.284,050 B2 Bagai (45) Date of Patent: Mar. 15, 2016 (54) AIRFOIL FOR ROTOR BLADE WITH (56) References Cited REDUCED PITCHING MOMENT U.S. PATENT
United States Patent (19)
 United States Patent (19) Fujita 11 Patent Number: (45) Date of Patent: 4,727,957 Mar. 1, 1988 (54) RUBBER VIBRATION ISOLATOR FOR MUFFLER 75 Inventor: Akio Fujita, Fujisawa, Japan 73) Assignee: Bridgestone
United States Patent (19) Fujita 11 Patent Number: (45) Date of Patent: 4,727,957 Mar. 1, 1988 (54) RUBBER VIBRATION ISOLATOR FOR MUFFLER 75 Inventor: Akio Fujita, Fujisawa, Japan 73) Assignee: Bridgestone
(12) United States Patent (10) Patent No.: US 9,475,637 B2
 US009475637B2 (12) United States Patent (10) Patent No.: US 9,475,637 B2 Perumal et al. (45) Date of Patent: Oct. 25, 2016 (54) PACKAGED ASSEMBLY FOR MACHINE 3,561,621 A * 2/1971 Rivers, Jr.... B6OP 1.00
US009475637B2 (12) United States Patent (10) Patent No.: US 9,475,637 B2 Perumal et al. (45) Date of Patent: Oct. 25, 2016 (54) PACKAGED ASSEMBLY FOR MACHINE 3,561,621 A * 2/1971 Rivers, Jr.... B6OP 1.00
(12) Patent Application Publication (10) Pub. No.: US 2015/ A1
 (19) United States US 20150224968A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0224968 A1 KM (43) Pub. Date: Aug. 13, 2015 (54) CONTROL METHOD FOR HILL START ASSIST CONTROL SYSTEM (71)
(19) United States US 20150224968A1 (12) Patent Application Publication (10) Pub. No.: US 2015/0224968 A1 KM (43) Pub. Date: Aug. 13, 2015 (54) CONTROL METHOD FOR HILL START ASSIST CONTROL SYSTEM (71)
od f 11 (12) United States Patent US 7,080,599 B2 Taylor Jul. 25, 2006 (45) Date of Patent: (10) Patent No.:
 US007080599B2 (12) United States Patent Taylor (10) Patent No.: (45) Date of Patent: Jul. 25, 2006 (54) RAILROAD HOPPER CAR TRANSVERSE DOOR ACTUATING MECHANISM (76) Inventor: Fred J. Taylor, 6485 Rogers
US007080599B2 (12) United States Patent Taylor (10) Patent No.: (45) Date of Patent: Jul. 25, 2006 (54) RAILROAD HOPPER CAR TRANSVERSE DOOR ACTUATING MECHANISM (76) Inventor: Fred J. Taylor, 6485 Rogers
(12) Patent Application Publication (10) Pub. No.: US 2007/ A1. Cervantes et al. (43) Pub. Date: Jun. 7, 2007
 US 20070 126577A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0126577 A1 Cervantes et al. (43) Pub. Date: Jun. 7, 2007 (54) DOOR LATCH POSITION SENSOR Publication Classification
US 20070 126577A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0126577 A1 Cervantes et al. (43) Pub. Date: Jun. 7, 2007 (54) DOOR LATCH POSITION SENSOR Publication Classification
USOO5963O14A United States Patent (19) 11 Patent Number: 5,963,014 Chen (45) Date of Patent: Oct. 5, 1999
 USOO5963O14A United States Patent (19) 11 Patent Number: 5,963,014 Chen (45) Date of Patent: Oct. 5, 1999 54 SERIALLY CONNECTED CHARGER Primary Examiner Edward H. Tso Attorney, Agent, or Firm-Rosenberger,
USOO5963O14A United States Patent (19) 11 Patent Number: 5,963,014 Chen (45) Date of Patent: Oct. 5, 1999 54 SERIALLY CONNECTED CHARGER Primary Examiner Edward H. Tso Attorney, Agent, or Firm-Rosenberger,
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0029246A1 Fratantonio et al. US 2008.0029246A1 (43) Pub. Date: (54) (75) (73) (21) (22) HEAT EXCHANGER BYPASS SYSTEM Inventors:
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0029246A1 Fratantonio et al. US 2008.0029246A1 (43) Pub. Date: (54) (75) (73) (21) (22) HEAT EXCHANGER BYPASS SYSTEM Inventors:
US 9, B2. Stamps et al. Jul. 11, (45) Date of Patent: (10) Patent No.: (12) United States Patent (54)
 US0097.02402B2 (12) United States Patent Stamps et al. (10) Patent No.: (45) Date of Patent: US 9,702.402 B2 Jul. 11, 2017 (54) (75) (73) (*) (21) (22) (65) (51) (52) (58) (56) INCREASED CAPACITY SPHERICAL
US0097.02402B2 (12) United States Patent Stamps et al. (10) Patent No.: (45) Date of Patent: US 9,702.402 B2 Jul. 11, 2017 (54) (75) (73) (*) (21) (22) (65) (51) (52) (58) (56) INCREASED CAPACITY SPHERICAL
(12) Patent Application Publication (10) Pub. No.: US 2004/ A1
 US 2004.00431 O2A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0043102 A1 H0 et al. (43) Pub. Date: Mar. 4, 2004 (54) ALIGNMENT COLLAR FOR A NOZZLE (52) U.S. Cl.... 425/567
US 2004.00431 O2A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0043102 A1 H0 et al. (43) Pub. Date: Mar. 4, 2004 (54) ALIGNMENT COLLAR FOR A NOZZLE (52) U.S. Cl.... 425/567
(12) United States Patent
 (12) United States Patent Swihla et al. USOO6287091B1 (10) Patent No.: (45) Date of Patent: US 6,287,091 B1 Sep. 11, 2001 (54) TURBOCHARGER WITH NOZZLE RING COUPLNG (75) Inventors: Gary R Svihla, Clarendon
(12) United States Patent Swihla et al. USOO6287091B1 (10) Patent No.: (45) Date of Patent: US 6,287,091 B1 Sep. 11, 2001 (54) TURBOCHARGER WITH NOZZLE RING COUPLNG (75) Inventors: Gary R Svihla, Clarendon
(12) Patent Application Publication (10) Pub. No.: US 2009/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0157272 A1 Uhler et al. US 2009015.7272A1 (43) Pub. Date: (54) (75) (73) (21) (22) (60) FOUR-PASSAGE MULTIFUNCTION TOROUE CONVERTER
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0157272 A1 Uhler et al. US 2009015.7272A1 (43) Pub. Date: (54) (75) (73) (21) (22) (60) FOUR-PASSAGE MULTIFUNCTION TOROUE CONVERTER
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States US 20080000052A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0000052 A1 Hong et al. (43) Pub. Date: Jan. 3, 2008 (54) REFRIGERATOR (75) Inventors: Dae Jin Hong, Jangseong-gun
(19) United States US 20080000052A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0000052 A1 Hong et al. (43) Pub. Date: Jan. 3, 2008 (54) REFRIGERATOR (75) Inventors: Dae Jin Hong, Jangseong-gun
(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0119926 A1 LIN US 2013 0119926A1 (43) Pub. Date: May 16, 2013 (54) WIRELESS CHARGING SYSTEMAND METHOD (71) Applicant: ACER
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0119926 A1 LIN US 2013 0119926A1 (43) Pub. Date: May 16, 2013 (54) WIRELESS CHARGING SYSTEMAND METHOD (71) Applicant: ACER
(12) United States Patent (10) Patent No.: US 7,125,133 B2
 US007125133B2 (12) United States Patent (10) Patent No.: US 7,125,133 B2 Bilotti et al. (45) Date of Patent: Oct. 24, 2006 (54) LED LIGHTING SYSTEM FOR PATIO 4.425,602 A 1/1984 Lansing UMBRELLA 5,053,931
US007125133B2 (12) United States Patent (10) Patent No.: US 7,125,133 B2 Bilotti et al. (45) Date of Patent: Oct. 24, 2006 (54) LED LIGHTING SYSTEM FOR PATIO 4.425,602 A 1/1984 Lansing UMBRELLA 5,053,931
HO (45) Date of Patent: Mar. 20, 2007
 (12) United States Patent US007191593B1 (10) Patent No.: US 7,191,593 B1 HO (45) Date of Patent: Mar. 20, 2007 (54) ELECTRO-HYDRAULIC ACTUATOR 5,072.584 A * 12/1991 Mauch et al.... 60/468 SYSTEM 5,351.914
(12) United States Patent US007191593B1 (10) Patent No.: US 7,191,593 B1 HO (45) Date of Patent: Mar. 20, 2007 (54) ELECTRO-HYDRAULIC ACTUATOR 5,072.584 A * 12/1991 Mauch et al.... 60/468 SYSTEM 5,351.914
(12) United States Patent
 US00704.4047B1 (12) United States Patent Bennett et al. (10) Patent No.: (45) Date of Patent: (54) (75) (73) (*) (21) (22) (51) (52) (58) CYLNDER MOUNTED STROKE CONTROL Inventors: Robert Edwin Bennett,
US00704.4047B1 (12) United States Patent Bennett et al. (10) Patent No.: (45) Date of Patent: (54) (75) (73) (*) (21) (22) (51) (52) (58) CYLNDER MOUNTED STROKE CONTROL Inventors: Robert Edwin Bennett,
Presented by. Navistar Education 2015
 Presented by Navistar Education 2015 1.2 Overview This course is intended to provide parts specialists with a description of Diesel Exhaust Fluid, or DEF, part number configuration, ordering and distribution
Presented by Navistar Education 2015 1.2 Overview This course is intended to provide parts specialists with a description of Diesel Exhaust Fluid, or DEF, part number configuration, ordering and distribution
November Jeffrey A. Wong Thomas L. Daugherty Gordon D. Huntzberry NOTICE
 Serial No. Filing Date Inventor 753.055 19 November 1996 Jeffrey A. Wong Thomas L. Daugherty Gordon D. Huntzberry NOTICE The above identified patent application is available for licensing. Requests for
Serial No. Filing Date Inventor 753.055 19 November 1996 Jeffrey A. Wong Thomas L. Daugherty Gordon D. Huntzberry NOTICE The above identified patent application is available for licensing. Requests for
(12) United States Patent Burkitt et a1.
 US008567174B2 (12) United States Patent Burkitt et a1. (10) Patent N0.: (45) Date of Patent: US 8,567,174 B2 Oct. 29, 2013 (54) (75) (73) (*) (21) (22) (86) (87) (65) (60) (51) (52) (58) VALVE ASSEMBLY
US008567174B2 (12) United States Patent Burkitt et a1. (10) Patent N0.: (45) Date of Patent: US 8,567,174 B2 Oct. 29, 2013 (54) (75) (73) (*) (21) (22) (86) (87) (65) (60) (51) (52) (58) VALVE ASSEMBLY
(12) United States Patent
 (12) United States Patent US00893 1520B2 (10) Patent No.: US 8,931,520 B2 Fernald (45) Date of Patent: Jan. 13, 2015 (54) PIPE WITH INTEGRATED PROCESS USPC... 138/104 MONITORING (58) Field of Classification
(12) United States Patent US00893 1520B2 (10) Patent No.: US 8,931,520 B2 Fernald (45) Date of Patent: Jan. 13, 2015 (54) PIPE WITH INTEGRATED PROCESS USPC... 138/104 MONITORING (58) Field of Classification
(12) Patent Application Publication (10) Pub. No.: US 2010/ A1
 US 20100300082A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0300082 A1 Zhang (43) Pub. Date: Dec. 2, 2010 (54) DIESEL PARTICULATE FILTER Publication Classification (51)
US 20100300082A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0300082 A1 Zhang (43) Pub. Date: Dec. 2, 2010 (54) DIESEL PARTICULATE FILTER Publication Classification (51)
(12) United States Patent (10) Patent No.: US 6,220,819 B1
 USOO6220819B1 (12) United States Patent (10) Patent No.: US 6,220,819 B1 Chien et al. (45) Date of Patent: Apr. 24, 2001 (54) CENTRIFUGAL PUMP IMPELLER 3.368,744 2/1968 Jenn... 416/237 4,236,871 12/1980
USOO6220819B1 (12) United States Patent (10) Patent No.: US 6,220,819 B1 Chien et al. (45) Date of Patent: Apr. 24, 2001 (54) CENTRIFUGAL PUMP IMPELLER 3.368,744 2/1968 Jenn... 416/237 4,236,871 12/1980
United States Patent (19) 11 Patent Number: 5,780,736 Russell 45) Date of Patent: Jul. 14, 1998
 III IIHIII USO05780736A O United States Patent (19) 11 Patent Number: 5,780,736 Russell 45) Date of Patent: Jul. 14, 1998 54 AXIAL THERMAL MASS FLOWMETER 3,733,897 5/1973 Herzl... 73/204.23 3,798,967 3/1974
III IIHIII USO05780736A O United States Patent (19) 11 Patent Number: 5,780,736 Russell 45) Date of Patent: Jul. 14, 1998 54 AXIAL THERMAL MASS FLOWMETER 3,733,897 5/1973 Herzl... 73/204.23 3,798,967 3/1974
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 2012O240592A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0240592 A1 Keny et al. (43) Pub. Date: Sep. 27, 2012 (54) COMBUSTOR WITH FUEL NOZZLE LINER HAVING CHEVRON
(19) United States US 2012O240592A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0240592 A1 Keny et al. (43) Pub. Date: Sep. 27, 2012 (54) COMBUSTOR WITH FUEL NOZZLE LINER HAVING CHEVRON
(12) United States Patent (10) Patent No.: US 8,511,619 B2
 USOO851 1619B2 (12) United States Patent (10) Patent No.: US 8,511,619 B2 Mann (45) Date of Patent: Aug. 20, 2013 (54) SLAT DEPLOYMENT MECHANISM (56) References Cited (75) Inventor: Alan Mann, Bristol
USOO851 1619B2 (12) United States Patent (10) Patent No.: US 8,511,619 B2 Mann (45) Date of Patent: Aug. 20, 2013 (54) SLAT DEPLOYMENT MECHANISM (56) References Cited (75) Inventor: Alan Mann, Bristol
(12) United States Patent
 US007307230B2 (12) United States Patent Chen (10) Patent No.: (45) Date of Patent: US 7,307,230 B2 Dec. 11, 2007 (54) MECHANISM FOR CONTROLLING CIRCUITCLOSINGAOPENING OF POWER RATCHET WRENCH (75) Inventor:
US007307230B2 (12) United States Patent Chen (10) Patent No.: (45) Date of Patent: US 7,307,230 B2 Dec. 11, 2007 (54) MECHANISM FOR CONTROLLING CIRCUITCLOSINGAOPENING OF POWER RATCHET WRENCH (75) Inventor:
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 201201.07098A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0107098 A1 Tirone, III et al. (43) Pub. Date: May 3, 2012 (54) GASTURBINE ENGINE ROTOR TIE SHAFT (52) U.S.
(19) United States US 201201.07098A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0107098 A1 Tirone, III et al. (43) Pub. Date: May 3, 2012 (54) GASTURBINE ENGINE ROTOR TIE SHAFT (52) U.S.
(12) United States Patent (10) Patent No.: US 8,840,124 B2
 USOO884O124B2 (12) United States Patent (10) Patent No.: Serhan et al. (45) Date of Patent: Sep. 23, 2014 (54) ROLLATOR HAVING ASITTO-LOCK BRAKE (56) References Cited (75) Inventors: Michael Serhan, Arcadia,
USOO884O124B2 (12) United States Patent (10) Patent No.: Serhan et al. (45) Date of Patent: Sep. 23, 2014 (54) ROLLATOR HAVING ASITTO-LOCK BRAKE (56) References Cited (75) Inventors: Michael Serhan, Arcadia,
Oxidation Technologies for Stationary Rich and Lean Burn Engines
 Oxidation Technologies for Stationary Rich and Lean Burn Engines ICAC MARAMA Advances in Air Pollution Control Technologies May 18-19, 2011 Baltimore, MD 1 Overview Oxidation catalyst technologies Oxidation
Oxidation Technologies for Stationary Rich and Lean Burn Engines ICAC MARAMA Advances in Air Pollution Control Technologies May 18-19, 2011 Baltimore, MD 1 Overview Oxidation catalyst technologies Oxidation
Electric motor pump with magnetic coupling and thrust balancing means
 Page 1 of 4 Electric motor pump with magnetic coupling and thrust balancing means Abstract ( 1 of 1 ) United States Patent 6,213,736 Weisser April 10, 2001 An electric motor pump for corrosive, electric
Page 1 of 4 Electric motor pump with magnetic coupling and thrust balancing means Abstract ( 1 of 1 ) United States Patent 6,213,736 Weisser April 10, 2001 An electric motor pump for corrosive, electric
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0018979 A1 McCoy et al. US 201200 18979A1 (43) Pub. Date: Jan. 26, 2012 (54) (76) (21) (22) (60) FIFTH WHEEL HITCH ISOLATION
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0018979 A1 McCoy et al. US 201200 18979A1 (43) Pub. Date: Jan. 26, 2012 (54) (76) (21) (22) (60) FIFTH WHEEL HITCH ISOLATION
Earl Sch yang y Lee, 5,457,342 10/1995 Herbst, II /712
 US005920264A United States Patent (19) 11 Patent Number: Kim et al. (45) Date of Patent: Jul. 6, 1999 54) COMPUTER SYSTEM PROTECTION 5,189,314 2/1993 Georgiou et al.... 307/271 DEVICE 5,287.292 2/1994
US005920264A United States Patent (19) 11 Patent Number: Kim et al. (45) Date of Patent: Jul. 6, 1999 54) COMPUTER SYSTEM PROTECTION 5,189,314 2/1993 Georgiou et al.... 307/271 DEVICE 5,287.292 2/1994
(12) United States Patent
 (12) United States Patent USOO8857684B1 (10) Patent No.: Calvert (45) Date of Patent: Oct. 14, 2014 (54) SLIDE-OUT TRUCK TOOL BOX (56) References Cited (71) Applicant: Slide Out Associates, Trustee for
(12) United States Patent USOO8857684B1 (10) Patent No.: Calvert (45) Date of Patent: Oct. 14, 2014 (54) SLIDE-OUT TRUCK TOOL BOX (56) References Cited (71) Applicant: Slide Out Associates, Trustee for
(12) United States Patent (10) Patent No.: US 6,929,039 B2
 USOO6929039B2 (12) United States Patent (10) Patent No.: US 6,929,039 B2 Vaitses () Date of Patent: Aug. 16, 2005 (54) MARINE VESSEL FUELOVERFLOW TANK 6,237,6 B1 5/2001 Pountney... 141/7 SYSTEM Primary
USOO6929039B2 (12) United States Patent (10) Patent No.: US 6,929,039 B2 Vaitses () Date of Patent: Aug. 16, 2005 (54) MARINE VESSEL FUELOVERFLOW TANK 6,237,6 B1 5/2001 Pountney... 141/7 SYSTEM Primary
(12) United States Patent
 (12) United States Patent Tomita et al. USOO6619259B2 (10) Patent No.: (45) Date of Patent: Sep. 16, 2003 (54) ELECTRONICALLY CONTROLLED THROTTLE CONTROL SYSTEM (75) Inventors: Tsugio Tomita, Hitachi (JP);
(12) United States Patent Tomita et al. USOO6619259B2 (10) Patent No.: (45) Date of Patent: Sep. 16, 2003 (54) ELECTRONICALLY CONTROLLED THROTTLE CONTROL SYSTEM (75) Inventors: Tsugio Tomita, Hitachi (JP);
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0091943 A1 Manor et al. US 2012009 1943A1 (43) Pub. Date: (54) (76) (21) (22) (86) (60) SOLAR CELL CHARGING CONTROL Inventors:
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0091943 A1 Manor et al. US 2012009 1943A1 (43) Pub. Date: (54) (76) (21) (22) (86) (60) SOLAR CELL CHARGING CONTROL Inventors:
of a quadratic function f(x)=aox+box+co whose con
 US005624250A United States Patent 19 11 Patent Number: 5,624,250 Son 45) Date of Patent: Apr. 29, 1997 54 TOOTH PROFILE FOR COMPRESSOR FOREIGN PATENT DOCUMENTS SCREW ROTORS 1197432 7/1970 United Kingdom.
US005624250A United States Patent 19 11 Patent Number: 5,624,250 Son 45) Date of Patent: Apr. 29, 1997 54 TOOTH PROFILE FOR COMPRESSOR FOREIGN PATENT DOCUMENTS SCREW ROTORS 1197432 7/1970 United Kingdom.
(12) Patent Application Publication (10) Pub. No.: US 2013/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0139355A1 Lee et al. US 2013 O1393.55A1 (43) Pub. Date: Jun. 6, 2013 (54) (75) (73) (21) (22) (60) HINGEMECHANISMAND FOLDABLE
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0139355A1 Lee et al. US 2013 O1393.55A1 (43) Pub. Date: Jun. 6, 2013 (54) (75) (73) (21) (22) (60) HINGEMECHANISMAND FOLDABLE
(12) United States Patent
 (12) United States Patent USOO9281614B1 (10) Patent No.: US 9.281,614 B1 Bonucci et al. (45) Date of Patent: Mar. 8, 2016 (54) CONNECTOR ASSEMBLY HAVING (56) References Cited LOCKING MEMBERS U.S. PATENT
(12) United States Patent USOO9281614B1 (10) Patent No.: US 9.281,614 B1 Bonucci et al. (45) Date of Patent: Mar. 8, 2016 (54) CONNECTOR ASSEMBLY HAVING (56) References Cited LOCKING MEMBERS U.S. PATENT
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0290354 A1 Marty et al. US 20140290354A1 (43) Pub. Date: Oct. 2, 2014 (54) (71) (72) (73) (21) (22) AIR DATA PROBE SENSE PORT
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0290354 A1 Marty et al. US 20140290354A1 (43) Pub. Date: Oct. 2, 2014 (54) (71) (72) (73) (21) (22) AIR DATA PROBE SENSE PORT
(12) United States Patent
 (12) United States Patent USOO698.1746B2 (10) Patent No.: US 6,981,746 B2 Chung et al. (45) Date of Patent: Jan. 3, 2006 (54) ROTATING CAR SEAT MECHANISM 4,844,543 A 7/1989 Ochiai... 297/344.26 4,925,227
(12) United States Patent USOO698.1746B2 (10) Patent No.: US 6,981,746 B2 Chung et al. (45) Date of Patent: Jan. 3, 2006 (54) ROTATING CAR SEAT MECHANISM 4,844,543 A 7/1989 Ochiai... 297/344.26 4,925,227
(12) United States Patent (10) Patent No.: US 6,435,993 B1. Tada (45) Date of Patent: Aug. 20, 2002
 USOO6435993B1 (12) United States Patent (10) Patent No.: US 6,435,993 B1 Tada (45) Date of Patent: Aug. 20, 2002 (54) HYDRAULIC CHAIN TENSIONER WITH 5,707.309 A 1/1998 Simpson... 474/110 VENT DEVICE AND
USOO6435993B1 (12) United States Patent (10) Patent No.: US 6,435,993 B1 Tada (45) Date of Patent: Aug. 20, 2002 (54) HYDRAULIC CHAIN TENSIONER WITH 5,707.309 A 1/1998 Simpson... 474/110 VENT DEVICE AND
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1. Lee et al. (43) Pub. Date: Mar. 9, 2006
 US 2006005 1222A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0051222 A1 Lee et al. (43) Pub. Date: Mar. 9, 2006 (54) MINIATURE PUMP FOR LIQUID COOLING Publication Classification
US 2006005 1222A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0051222 A1 Lee et al. (43) Pub. Date: Mar. 9, 2006 (54) MINIATURE PUMP FOR LIQUID COOLING Publication Classification
(12) United States Patent
 (12) United States Patent USOO7242106B2 (10) Patent No.: US 7,242,106 B2 Kelly (45) Date of Patent: Jul. 10, 2007 (54) METHOD OF OPERATION FOR A (56) References Cited SE NYAVE ENERGY U.S. PATENT DOCUMENTS
(12) United States Patent USOO7242106B2 (10) Patent No.: US 7,242,106 B2 Kelly (45) Date of Patent: Jul. 10, 2007 (54) METHOD OF OPERATION FOR A (56) References Cited SE NYAVE ENERGY U.S. PATENT DOCUMENTS
United States Patent (19) Kim et al.
 United States Patent (19) Kim et al. 54 METHOD OF AND APPARATUS FOR COATING AWAFER WITH A MINIMAL LAYER OF PHOTORESIST 75 Inventors: Moon-woo Kim, Kyungki-do; Byung-joo Youn, Seoul, both of Rep. of Korea
United States Patent (19) Kim et al. 54 METHOD OF AND APPARATUS FOR COATING AWAFER WITH A MINIMAL LAYER OF PHOTORESIST 75 Inventors: Moon-woo Kim, Kyungki-do; Byung-joo Youn, Seoul, both of Rep. of Korea
United States Patent (19) Muranishi
 United States Patent (19) Muranishi (54) DEVICE OF PREVENTING REVERSE TRANSMISSION OF MOTION IN A GEAR TRAIN 75) Inventor: Kenichi Muranishi, Ena, Japan 73) Assignee: Ricoh Watch Co., Ltd., Nagoya, Japan
United States Patent (19) Muranishi (54) DEVICE OF PREVENTING REVERSE TRANSMISSION OF MOTION IN A GEAR TRAIN 75) Inventor: Kenichi Muranishi, Ena, Japan 73) Assignee: Ricoh Watch Co., Ltd., Nagoya, Japan
(12) United States Patent (10) Patent No.: US 8.499,556 B2
 US008499.556B2 (12) United States Patent () Patent No.: US 8.499,556 B2 Henriksson et al. (45) Date of Patent: Aug. 6, 2013 (54) EXHAUST PURIFICATION SYSTEM WITH A (56) References Cited DESEL PARTICULATE
US008499.556B2 (12) United States Patent () Patent No.: US 8.499,556 B2 Henriksson et al. (45) Date of Patent: Aug. 6, 2013 (54) EXHAUST PURIFICATION SYSTEM WITH A (56) References Cited DESEL PARTICULATE
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1
 (19) United States US 2012O324985A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0324985 A1 Gu et al. (43) Pub. Date: (54) FLUID LEAK DETECTION SYSTEM (52) U.S. Cl.... 73A4OS R (75) Inventors:
(19) United States US 2012O324985A1 (12) Patent Application Publication (10) Pub. No.: US 2012/0324985 A1 Gu et al. (43) Pub. Date: (54) FLUID LEAK DETECTION SYSTEM (52) U.S. Cl.... 73A4OS R (75) Inventors:
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States US 201401 11961A1 (12) Patent Application Publication (10) Pub. No.: US 2014/011 1961 A1 Liu et al. (43) Pub. Date: Apr. 24, 2014 (54) WIRELESS BROADBAND DEVICE Publication Classification
(19) United States US 201401 11961A1 (12) Patent Application Publication (10) Pub. No.: US 2014/011 1961 A1 Liu et al. (43) Pub. Date: Apr. 24, 2014 (54) WIRELESS BROADBAND DEVICE Publication Classification
(12) United States Patent
 (12) United States Patent USO09599540B2 (10) Patent No.: Kim (45) Date of Patent: Mar. 21, 2017 (54) SYSTEM AND METHOD FOR MEASURING 4,112,630 A * 9/1978 Brown, Jr.... B24B 5,366 CONICITY USING FOUR FORCE-SENSORS
(12) United States Patent USO09599540B2 (10) Patent No.: Kim (45) Date of Patent: Mar. 21, 2017 (54) SYSTEM AND METHOD FOR MEASURING 4,112,630 A * 9/1978 Brown, Jr.... B24B 5,366 CONICITY USING FOUR FORCE-SENSORS
(12) United States Patent
 (12) United States Patent US007884512B2 (10) Patent No.: US 7,884,512 B2 Horng et al. (45) Date of Patent: Feb. 8, 2011 (54) FIXING STRUCTURE FOR PRINTED (56) References Cited CIRCUIT BOARD OF MICRO MOTOR
(12) United States Patent US007884512B2 (10) Patent No.: US 7,884,512 B2 Horng et al. (45) Date of Patent: Feb. 8, 2011 (54) FIXING STRUCTURE FOR PRINTED (56) References Cited CIRCUIT BOARD OF MICRO MOTOR
(12) United States Patent (10) Patent No.: US 6,446,482 B1. Heskey et al. (45) Date of Patent: Sep. 10, 2002
 USOO64.46482B1 (12) United States Patent (10) Patent No.: Heskey et al. (45) Date of Patent: Sep. 10, 2002 (54) BATTERY OPERATED HYDRAULIC D408.242 S 4/1999 Yamamoto... D8/61 COMPRESSION TOOL WITH RAPID
USOO64.46482B1 (12) United States Patent (10) Patent No.: Heskey et al. (45) Date of Patent: Sep. 10, 2002 (54) BATTERY OPERATED HYDRAULIC D408.242 S 4/1999 Yamamoto... D8/61 COMPRESSION TOOL WITH RAPID
(12) Patent Application Publication (10) Pub. No.: US 2014/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0018203A1 HUANG et al. US 20140018203A1 (43) Pub. Date: Jan. 16, 2014 (54) (71) (72) (73) (21) (22) (30) TWO-STAGE DIFFERENTIAL
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0018203A1 HUANG et al. US 20140018203A1 (43) Pub. Date: Jan. 16, 2014 (54) (71) (72) (73) (21) (22) (30) TWO-STAGE DIFFERENTIAL
(12) Patent Application Publication (10) Pub. No.: US 2010/ A1
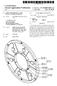 (19) United States US 2010O231027A1 (12) Patent Application Publication (10) Pub. No.: US 2010/0231027 A1 SU (43) Pub. Date: Sep. 16, 2010 (54) WHEEL WITH THERMOELECTRIC (30) Foreign Application Priority
(19) United States US 2010O231027A1 (12) Patent Application Publication (10) Pub. No.: US 2010/0231027 A1 SU (43) Pub. Date: Sep. 16, 2010 (54) WHEEL WITH THERMOELECTRIC (30) Foreign Application Priority
(12) United States Patent (10) Patent No.: US 8.408,189 B2
 USOO8408189B2 (12) United States Patent () Patent No.: US 8.408,189 B2 Lutz et al. (45) Date of Patent: Apr. 2, 2013 (54) PETROL ENGINE HAVING A LOW-PRESSURE EGR CIRCUIT (56) References Cited U.S. PATENT
USOO8408189B2 (12) United States Patent () Patent No.: US 8.408,189 B2 Lutz et al. (45) Date of Patent: Apr. 2, 2013 (54) PETROL ENGINE HAVING A LOW-PRESSURE EGR CIRCUIT (56) References Cited U.S. PATENT
E. E. E.O.E. comprises a diverter valve downstream of the turbine, an
 USOO63056B1 (12) United States Patent (10) Patent No.: Lui (45) Date of Patent: Oct. 23, 2001 (54) INTEGRATED BLEED AIR AND ENGINE 5,363,641 11/1994 Dixon et al.. STARTING SYSTEM 5,414,992 5/1995 Glickstein.
USOO63056B1 (12) United States Patent (10) Patent No.: Lui (45) Date of Patent: Oct. 23, 2001 (54) INTEGRATED BLEED AIR AND ENGINE 5,363,641 11/1994 Dixon et al.. STARTING SYSTEM 5,414,992 5/1995 Glickstein.
(12) (10) Patent No.: US 6,915,721 B2. Hsu et al. (45) Date of Patent: Jul. 12, 2005
 United States Patent USOO6915721B2 (12) (10) Patent No.: US 6,915,721 B2 Hsu et al. (45) Date of Patent: Jul. 12, 2005 (54) CORDLESS RATCHET WRENCH 6,311,583 B1 11/2001 Izumisawa... 81/57.13 6,715,380
United States Patent USOO6915721B2 (12) (10) Patent No.: US 6,915,721 B2 Hsu et al. (45) Date of Patent: Jul. 12, 2005 (54) CORDLESS RATCHET WRENCH 6,311,583 B1 11/2001 Izumisawa... 81/57.13 6,715,380
(12) Patent Application Publication (10) Pub. No.: US 2012/ A1. Poulsen (43) Pub. Date: Oct. 25, 2012
 US 20120268067A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0268067 A1 Poulsen (43) Pub. Date: (54) CHARGING STATION FOR ELECTRIC (52) U.S. Cl.... 320/109; 29/401.1 VEHICLES
US 20120268067A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0268067 A1 Poulsen (43) Pub. Date: (54) CHARGING STATION FOR ELECTRIC (52) U.S. Cl.... 320/109; 29/401.1 VEHICLES
United States Patent (19)
 United States Patent (19) USOO5287906A 11 Patent Number: 5,287,906 Stech (45) Date of Patent: Feb. 22, 1994 54 AIR CONTROL SYSTEM FOR PNEUMATIC 3,100,6 8/1963 Work... 285/33 TRES ON A WEHICLE 4,387,931
United States Patent (19) USOO5287906A 11 Patent Number: 5,287,906 Stech (45) Date of Patent: Feb. 22, 1994 54 AIR CONTROL SYSTEM FOR PNEUMATIC 3,100,6 8/1963 Work... 285/33 TRES ON A WEHICLE 4,387,931
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 (19) United States US 2011 0183181A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0183181 A1 M00n et al. (43) Pub. Date: Jul. 28, 2011 (54) SECONDARY BATTERY HAVING NSULATION BAG (76) Inventors:
(19) United States US 2011 0183181A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0183181 A1 M00n et al. (43) Pub. Date: Jul. 28, 2011 (54) SECONDARY BATTERY HAVING NSULATION BAG (76) Inventors:
United States Patent (19) Reid
 United States Patent (19) Reid 54 76) 21 22 (51) 52) 58 56) CONVENIENT DUAL FUELTANK SYSTEM Inventor: Richard M. Reid, 25474 State St., Loma Linda, Calif. 92354 Appl. No.: 638,377 Filed: Aug. 7, 1984 Int.
United States Patent (19) Reid 54 76) 21 22 (51) 52) 58 56) CONVENIENT DUAL FUELTANK SYSTEM Inventor: Richard M. Reid, 25474 State St., Loma Linda, Calif. 92354 Appl. No.: 638,377 Filed: Aug. 7, 1984 Int.
(12) United States Patent
 USOO8545166 B2 (12) United States Patent Maruthamuthu et al. (10) Patent No.: (45) Date of Patent: Oct. 1, 2013 (54) (75) (73) (*) (21) (22) (65) (51) (52) (58) SYSTEMAND METHOD FOR CONTROLLING LEAK STEAM
USOO8545166 B2 (12) United States Patent Maruthamuthu et al. (10) Patent No.: (45) Date of Patent: Oct. 1, 2013 (54) (75) (73) (*) (21) (22) (65) (51) (52) (58) SYSTEMAND METHOD FOR CONTROLLING LEAK STEAM
United States Patent 19 Schechter
 United States Patent 19 Schechter (54) 75 73) 21) (22) (51) (52) 58 (56) SPOOL VALVE CONTROL OF AN ELECTROHYDRAULIC CAMILESS WALVETRAIN Inventor: Michael M. Schechter, Farmington Hills, Mich. Assignee:
United States Patent 19 Schechter (54) 75 73) 21) (22) (51) (52) 58 (56) SPOOL VALVE CONTROL OF AN ELECTROHYDRAULIC CAMILESS WALVETRAIN Inventor: Michael M. Schechter, Farmington Hills, Mich. Assignee:
(12) United States Patent
 USOO861 8656B2 (12) United States Patent Oh et al. (54) FLEXIBLE SEMICONDUCTOR PACKAGE APPARATUS HAVING ARESPONSIVE BENDABLE CONDUCTIVE WIRE MEMBER AND A MANUFACTURING THE SAME (75) Inventors: Tac Keun.
USOO861 8656B2 (12) United States Patent Oh et al. (54) FLEXIBLE SEMICONDUCTOR PACKAGE APPARATUS HAVING ARESPONSIVE BENDABLE CONDUCTIVE WIRE MEMBER AND A MANUFACTURING THE SAME (75) Inventors: Tac Keun.
(12) United States Patent (10) Patent No.: US 6,543,270 B2
 USOO654327OB2 (12) United States Patent (10) Patent No.: US 6,543,270 B2 Cmelik (45) Date of Patent: Apr. 8, 2003 (54) AUTOBODY DENT REPAIR TOOL 4,461,192 A * 7/1984 Suligoy et al.... 81/177.7 4,502,317
USOO654327OB2 (12) United States Patent (10) Patent No.: US 6,543,270 B2 Cmelik (45) Date of Patent: Apr. 8, 2003 (54) AUTOBODY DENT REPAIR TOOL 4,461,192 A * 7/1984 Suligoy et al.... 81/177.7 4,502,317
