FLAME STUDIES ON CONVENTIONAL, ALTERNATIVE, AND SURROGATE JET FUELS, AND THEIR REFERENCE HYDROCARBONS
|
|
|
- Lucy Amber Elliott
- 6 years ago
- Views:
Transcription
1 FLAME STUDIES ON CONVENTIONAL, ALTERNATIVE, AND SURROGATE JET FUELS, AND THEIR REFERENCE HYDROCARBONS by XIN HUI Submitted in partial fulfilment of the requirements For the degree of Doctor of Philosophy Dissertation Advisor: Dr. Chih-Jen Sung Department of Mechanical and Aerospace Engineering Case Western Reserve University January, 2013
2
3 Table of Contents Table of Contents...i List of Tables...iv List of Figures... v Acknowledgements... xiii Chapter 1 Introduction Overview and Significance Combustion Responses Scopes and Objectives Overview of Dissertation... 7 Chapter 2 Experimental Specifications Experimental Setup High Pressure Counterflow Burner Mixture Preparation DPIV Measurement Laminar Flame Speed Determination Counterflow Twin-Flame Configuration Linear and Nonlinear Extrapolations Extinction Stretch Rate Determination Chapter 3 Computational Specifications Flame Propagation Extinction Limit Mixture-averaged and Multicomponent Transport Properties i -
4 Chapter 4 Flame Propagation and Extinction of Jet-A and Alternative Jet Fuels Background Kinetic Models Results Sensitivity Analysis Derived Cetane Number Summary Chapter 5 Flame Propagation and Extinction of Jet-A and Surrogate Fuels Background Surrogate Formulation Concept of Distinct Chemical Functionality Combustion Property Targets Surrogate Component Selection Results Summary Chapter 6 Flame Propagation and Extinction of Selected Aromatic Hydrocarbons Background Kinetic Models Results Sensitivity and Flux Analysis Summary ii -
5 Chapter 7 Flame Propagation of Liquid Hydrocarbon Fuels at Elevated Pressures Background Experimental Conditions Kinetic Models Results Summary Chapter 8 Summary and Recommendations Summary Recommendations References iii -
6 List of Tables Table 2.1 Specifications of gases and fuels...11 Table 4.1 Physical properties of Jet-A and alternative jet fuels Table 4.2 Measured ignition delays, Derived Cetane Numbers, and Cetane Numbers of various jet fuels Table 5.1 Surrogate component candidates selected for the MURI research Table 5.2 Combustion property targets of Jet-A, 1 st and 2 nd generation MURI surrogates Table 6.1 Molecular structures of the aromatic components investigated iv -
7 List of Figures Figure 2.1 Schematic of the flow control system, high pressure counterflow burner setup, and DPIV system Figure 2.2 (a) High pressure chamber; (b) top counterflow burner Figure 2.3 (a) Schematic of PIV measurement principle (Dantec, 2006); (b) images and velocity maps with and without flames Figure 2.4 (a) Schematic of the counterflow twin flame configuration; (b) axial velocity profile along central line (simulated) Figure 2.5 PIV measured velocity profile, (a) axial velocity; (b) radial velocity Figure 2.6 Reference flame speed of n-pb/air mixtures at an unburned mixture temperature of T =400 K and equivalence ratios of =0.7, 1.0, and 1.4, along with the demonstration of linear (solid lines) and nonlinear (dashed lines) extrapolations, (a) reference flame speed versus stretch rate; (b) reference flame speed versus Karlovitz number Figure 3.1 (a) Computed axial velocity and temperature profiles and (b) computed flame response curve Figure 3.2 Differences between mixture-averaged formulation and multicomponent formulation in laminar flame speed and extinction limit simulations for 1,2,4-TMB flames at T =400 K and P=1 atm Figure 4.1 (a) Distribution of hydrocarbons in five SPK fuels (Moses, 2008) and (b) chromatograms of conventional and alternative jet fuels with n- paraffins identified v -
8 Figure 4.2 Reference flame speeds versus stretch rates for Jet-A/air, S-8/air, IPK/air, and camelina/air mixtures at 400 K preheat temperature and equivalence ratios of =0.8, 1.0, and Figure 4.3 Laminar flame speeds of Jet-A/air, S-8/air, IPK/air, and camelina/air mixtures as a function of equivalence ratio at preheat temperatures of (a) 400 K and (b) 470 K Figure 4.4 Experimental measured and computed values of laminar flame speeds of Jet-A/air mixtures as a function of equivalence ratio at preheat temperatures of 400 K and 470 K Figure 4.5 Experimental measured and computed values of laminar flame speeds of S-8/air mixtures as a function of equivalence ratio at preheat temperatures of 400 K and 470 K Figure 4.6 Laminar flame speeds of Jet-A/air, S-8/air, and n-decane/air mixtures as a function of equivalence ratio at preheat temperatures of 400 K and 470 K Figure 4.7 Extinction stretch rates of Jet-A/oxidizer, S-8/oxidizer, IPK/oxidizer, and camelina/oxidizer mixtures as a function of equivalence ratio at preheat temperatures of (a) 400 K and (b) 470 K Figure 4.8 (a) Computed maximum flame temperature response to stretch rate variations and (b) comparison of experimental and computed extinction stretch rates for Jet-A flames at a preheat temperature of 400 K Figure 4.9 Comparative extinction stretch rates for Jet-A/oxidizer, S-8/oxidizer, n-decane/oxidizer, and n-dodecane/oxidizer mixtures at a preheat temperature of 400 K vi -
9 Figure 4.10 Normalized sensitivity coefficients of Jet-A/air mixtures for (a) laminar burning flux and (b) extinction stretch rate Figure 4.11 (a)-(c) Measured ignition delays and Derived Cetane Numbers of binary fuel blends in accordance with ASTM D7170, (d) measured Derived Cetane Numbers of binary fuel blends based on ASTM D6890 taken from the study of Bessee et al. (2011) Figure 5.1 World-wide average molecular class distribution of Jet-A (Shafer et al., 2006) Figure 5.2 Schematic diagram of real fuel oxidation and concept of distinct chemical functionality (Dooley et al., 2012) Figure 5.3 Comparison of laminar flame speeds of Jet-A, 1 st and 2 nd generation MURI surrogates at preheat temperatures of (a) 400 K and (b) 470 K.. 79 Figure 5.4 Comparison of extinction stretch rates of Jet-A, 1 st and 2 nd generation MURI surrogates at preheat temperatures of (a) 400 K, and (b) 470 K Figure 6.1 Comparison of laminar flame speeds of toluene/air, n-pb/air, 1,2,4- TMB/air, and 1,3,5-TMB/air mixtures at unburned mixture temperatures of (a) T =400 K and (b) T =470 K Figure 6.2 Computed adiabatic flame temperatures of toluene/air, n-pb/air, and 1,2,4-TMB/air mixtures at T =400 K and P=1 atm Figure 6.3 Pictures of near-extinction flames of n-pb/oxidizer mixtures: (a) lean flame of =0.9 (Le=2.97) and (b) rich flame of =1.2 (Le=0.95) Figure 6.4 Comparison of extinction stretch rates of toluene/oxidizer, n- PB/oxidizer, 1,2,4-TMB/oxidizer, and 1,3,5-TMB/oxidizer mixtures at an unburned mixture temperature of T =400 K and P=1 atm vii -
10 Figure 6.5 Comparison of experimental and computed laminar flame speeds of toluene/air mixtures at unburned mixture temperatures of T =400 and 470 K, and P=1 atm Figure 6.6 Comparison of experimental and computed laminar flame speeds of n- PB/air mixtures at unburned mixture temperatures of T =400 and 470 K, and P=1 atm Figure 6.7 Comparison of experimental and computed laminar flame speeds of 1,2,4-TMB/air mixtures at unburned mixture temperatures of T =400 and 470 K, and P= 1atm Figure 6.8 Comparison of experimental and computed extinction stretch rates of toluene/oxidizer mixtures at an unburned mixture temperature of T =400 K and P=1 atm Figure 6.9 Comparison of experimental and computed extinction stretch rates of n-pb/oxidizer mixtures at an unburned mixture temperature of T =400 K and P=1 atm Figure 6.10 Comparison of experimental and computed extinction stretch rates of 1,2,4-TMB/oxidizer mixtures at an unburned mixture temperature of T =400 K and P=1 atm Figure 6.11 Normalized sensitivity coefficients of reaction rates for mass burning flux and extinction limit of toluene/oxidizer mixture at T =400 K, =1, and P=1 atm Figure 6.12 Normalized sensitivity coefficients of reaction rates for mass burning flux and extinction limit of n-pb/oxidizer mixture at T =400 K, =1, and P=1 atm viii -
11 Figure 6.13 Normalized sensitivity coefficients of reaction rates for mass burning flux and extinction limit of 1,2,4-TMB/oxidizer mixture at T =400 K, =1, and P=1 atm Figure 6.14 Reaction path analysis for the n-pb freely-propagating and nearextinction flames at T =400 K, =1.0, and P=1 atm Figure 6.15 Spatially-resolved mass fraction profiles of combined C 2 H 2 and C 2 H 4 radicals in the freely propagating toluene/air, n-pb/air, and 1,2,4- TMB/air flames at T =400 K, =1.0, and P=1 atm Figure 6.16 Spatially-resolved mass fraction profiles of benzylic radicals in the freely propagating toluene/air, n-pb/air, and 1,2,4-TMB/air flames at T =400 K, =1.0, and P=1 atm Figure 7.1 Saturation curves for some typical large hydrocarbons. Data are taken from NIST Figure 7.2 Experimental data (symbols) showing reference flame speed versus Karlovitz number for (a) n-decane/air mixtures and (b) Jet-A/air mixtures at a preheat temperature of T =400 K, an equivalence ratio of =1.0, and pressures of P=1 3 atm, along with the demonstration of linear (dashed lines) and nonlinear extrapolations (solid lines) Figure 7.3 Laminar flame speeds of n-decane/air mixtures at a preheat temperature of T =400 K and pressures of P=1 3 atm as a function of equivalence ratio. Symbols: experimental data; lines: simulated results Figure 7.4 Laminar flame speeds of n-dodecane/air mixtures at a preheat temperature of T =400 K and pressures of P=1 3 atm as a function - ix -
12 of equivalence ratio. Symbols: experimental data; lines: simulated results Figure 7.5 Laminar flame speeds of iso-octane/air mixtures at a preheat temperature of T =400 K and pressures of P=1 3 atm as a function of equivalence ratio. Symbols: experimental data; lines: simulated results Figure 7.6 Laminar flame speeds of toluene/air mixtures at a preheat temperature of T =400 K and pressures of P=1 3 atm as a function of equivalence ratio. Symbols: experimental data; lines: simulated results Figure 7.7 Laminar flame speeds of n-pb/air mixtures at a preheat temperature of T =400 K and pressures of P=1 3 atm as a function of equivalence ratio. Symbols: experimental data; lines: simulated results Figure 7.8 Laminar flame speeds of 1,2,4-TMB/air mixtures at a preheat temperature of T =400 K and pressures of P=1 3 atm as a function of equivalence ratio. Symbols: experimental data; lines: simulated results Figure 7.9 Comparison of experimentally determined laminar flame speeds of 1,3,5-TMB/air mixtures (filled symbols) and 1,2,4-TMB/air mixtures (open symbols) at a preheat temperature of T =400 K and pressures of P=1 3 atm Figure 7.10 Laminar flame speeds of Jet-A/air mixtures at a preheat temperature of T =400 K and pressures of P =1 3 atm as a function of equivalence ratio. Symbols: experimental data; lines: simulated results x -
13 Figure 7.11 Laminar flame speeds of S-8/air mixtures (filled symbols) and Jet- A/air mixtures (open symbols) at a preheat temperature of T =400 K and pressures of P =1 3 atm. Symbols: experimental data; line: simulated results Figure 7.12 Comparison of experimentally determined laminar flame speeds of Jet-A/air mixtures (filled symbols) and Jet-A surrogate/air mixtures (open symbols) at a preheat temperature of T =400 K and pressures of P=1 3 atm. The 2 nd generation MURI surrogate is proposed by Dooley et al. (2012) Figure 7.13 Experimentally-determined laminar flame speeds of stoichiometric n- decane/air, iso-octane/air, toluene/air, and Jet-A/air mixtures at pressures of (a) P=1 atm and (b) P=2 atm as a function of preheat temperature Figure 7.14 Experimentally-determined laminar burning flux of (a) n-decane/air mixtures and (b) Jet-A/air mixtures at a preheat temperature of T =400 K and pressures of P=1 3 atm as a function of equivalence ratio Figure 7.15 Experimentally-determined laminar burning flux of n-decane/air mixtures at a preheat temperature of T =400 K and equivalence ratios of =0.7, 1.0, and 1.3 as a function of pressure Figure 7.16 Experimentally-determined laminar burning flux of n-dodecane/air mixtures at a preheat temperature of T =400 K and equivalence ratios of =0.7, 1.0, and 1.3 as a function of pressure xi -
14 Figure 7.17 Experimentally-determined laminar burning flux of iso-octane/air mixtures at a preheat temperature of T =400 K and equivalence ratios of =0.7, 1.0, and 1.3 as a function of pressure Figure 7.18 Experimentally-determined laminar burning flux of toluene/air mixtures at a preheat temperature of T =400 K and equivalence ratios of =0.7, 1.0, and 1.3 as a function of pressure Figure 7.19 Experimentally-determined laminar burning flux of 1,2,4-TMB/air mixtures at a preheat temperature of T =400 K and equivalence ratios of =0.7, 1.0, and 1.3 as a function of pressure Figure 7.20 Experimentally-determined laminar burning flux of n-pb/air mixtures at a preheat temperature of T =400 K and equivalence ratios of =0.7, 1.0, and 1.3 as a function of pressure Figure 7.21 Experimentally-determined laminar burning flux of Jet-A/air mixtures at a preheat temperature of T =400 K and equivalence ratios of =0.7, 1.0, and 1.3 as a function of pressure Figure 7.22 Experimentally-determined laminar burning flux of S-8/air mixtures at a preheat temperature of T =400 K and equivalence ratios of =0.7, 1.0, and 1.3 as a function of pressure Figure 7.23 Experimentally-extracted overall reaction orders of various fuel/air mixtures as a function of equivalence ratio at a preheat temperature of T =400 K xii -
15 Acknowledgements I would like, first of all, to acknowledge and thank my advisor Dr. Chih-Jen Sung for his guidance, patience, and constant support, and for sharing his extensive knowledge of and very contagious passion for combustion research during the process as I have pursued my Ph.D. degree. I would like to acknowledge the Air Force Office of Scientific Research for its sponsorship and support of this work. I would like to acknowledge and thank Dylan Gardner for providing me the derived cetane number data for conventional and alternative jet fuels. I would also like to thank Dr. Kamal Kumar for his invaluable help and advice. Through his guidance and patience, I was able to familiarize myself with the techniques of laminar flame speed measurement and simulation. I am also very grateful to my colleague Dr. Apurba K. Das for all his time, patience, and tireless support. With his inspiring and encouraging, I was able to overcome many difficulties and finally see a light at the end of the tunnel. Many thanks to my lab mates, Kyle Brady, Bryan Weber, Pradeep K. Singh, and Goutham Kukkadapu for their support, help, ideas, discussions, and good times that we have spent together. Finally, I would like to thank my parents Achun Hui, and Fengju Xi for always supporting me and believing in me, and a special thank goes to my wife Cui Tao for accompanying me during my hardest time. - xiii -
16 Flame Studies on Conventional, Alternative, and Surrogate Jet Fuels, and Their Reference Hydrocarbons. Abstract by XIN HUI This dissertation presents work on the flame propagation and extinction of various liquid hydrocarbon fuels, including conventional and alternative jet fuels, surrogate fuels, and their reference hydrocarbon components. The laminar flame speeds and extinction stretch rates are experimentally determined by using a twin-flame counterflow setup integrated with a Digital Particle Image Velocimetry system for the flow field measurement. The experimental results are also compared with computed values obtained by using various published kinetic models for different fuels. In general, most of the simulation results agree with the experimental data with an average deviation less than 10%, which are reasonable considering the uncertainties in both experiments and kinetic models. The results of this work show that the conventional Jet-A and alternative jet fuels share very similar flame speeds and extinction limits despite of their differences in the molecular composition. The results of two surrogate mixtures for Jet-A show that they are both able to reproduce very well the flame speeds and extinction limits of the target jet fuel. Additional studies on aromatic species relevant to the conventional jet fuels illustrate that the degree and position of alkyl substitution on the benzene ring have a strong effect on the reactivity of the aromatic components studied. By extending the flame propagation studies to - xiv -
17 elevated pressures up to 3 atm, it is found that the flame speed results at elevated pressures are consistent in the trend with atmospheric results. Further attempts are made to identify and quantify the effects of preheat temperature and pressure on burning rate. This dissertation provides experimental flame speed and extinction data of high fidelity for jet fuels and relevant hydrocarbons. The fundamental data provided herein can serve as the benchmark database, and can be used in development and validation of combustion kinetic models. - xv -
18 Chapter 1 Introduction 1.1 Overview and Significance Global energy demand will continue to increase in the next decade as the world has been reeling from the financial crisis and economic recession. As much as 70% of the world s energy comes from the consumption of crude oil and natural gas. It is expected that liquid hydrocarbons will continue to dominate the transportation sector due to their high energy density and liquid form which makes them easy to handle and transport. The air transport has grown faster than any other transport mode in recent years and is likely to continue growing rapidly in the future. The efficiency of air transport has been improving steadily over time as airlines respond to high fuel costs, but at a much slower rate than travel growth. As a consequence, with air transport growing rapidly it is also eliciting growing concerns due to its environmental impact and its vulnerability with respect to energy security. These issues have put the aviation sector at the forefront of tide in achieving energy efficiency. Efforts have been made on very front to improve efficiency and reduce emissions through better technology, optimized operation, as well energy-saving infrastructure (The World Bank, 2012). Aviation fuel is a specialized type of petroleum-based fuel to power aircraft. It is generally of a higher quality than fuels used in less critical applications, such as heating or road transport, and often contains additives to reduce the risk of icing or explosion. Aviation fuel can be categorized into aviation gasoline and kerosene jet fuel. In 1950 aviation was almost entirely gasoline powered; by 2000 it was 99% jet fuel powered. The growth in the use of aviation fuel is illustrated by the fact that aviation fuel was only 3% of petroleum consumption for transportation in 1950, but had grown to 12% in 1965 and has maintained that share since then (Behrens and - 1 -
19 Glover, 2012). Today, the aviation sector burns about 5.8% of total oil consumed in the world, which is around 5 million barrels per day, and the demand for aviation fuels is expected to be doubled over the next thirty years (ExxonMobil, 2012). The ever-increasing demand for petroleum-based fuels coupled with the finite fossil energy resources has led to a great interest in the development of the nonpetroleum-based, renewable energy. In aviation sector, concerns about rising fuel price, energy supply, energy security, climate change, and aviation emissions call for a fresh look at the use of the alternative jet fuels. Alternative jet fuels derived from coal, biomass, tar sand, and oil shale are desirable both strategically and more generally in terms of reducing dependence on petroleum. Researchers have been looking into ways to power both military and commercial aircrafts with alternative jet fuels (Blackwell, 2007), although many caution that widespread use could be years or even decades away. In addition to reducing petroleum reliance, alternative fuels should also become a major driver in reaching the objective of carbon-neutral growth for aviation. Recently, drop-in bio-jet fuels have been successfully tested and are already in use on certain commercial routes. The industry is aiming at replacing 6% of current jet fuels with bio-jet fuels by However, beyond the environmental issue, the major challenge will be the assessment of life cycle effects associated with production and use of an alternative fuel. Aircraft and engine companies are currently investigating alternative jet fuels that can be blended with, or completely replace conventional jet fuels without necessitating any substantial modifications to engine or aircraft. In another word, the alternative fuels should not only have comparable physical and chemical properties, but also exhibit similar combustion characteristics and further engine performance with petroleum-derived jet fuels. Since alternative fuels can be derived from different - 2 -
20 sources, the blending of alternative fuels with petroleum fuels as well as their long term potential substitution for petroleum products heightens the need to understand the relation of fuel composition to operational properties as well as to fundamental combustion chemistry (Edwards et al., 2010). Considerable work has been devoted to the studies of practical jet fuels. The ultimate goal for the fundamental research is to develop and validate physics-based models to enable quantitative emissions and performance predictions using combustion modelling, which is not only necessary to assess the feasibility of alternative fuels in aviation applications, but also important in developing high-efficiency and clean-burning aeropropulsion engines of the next generation. Jet fuels including conventional and alternative jet fuels are mixtures of hundreds of different hydrocarbons from different molecular classes (ExxonMobil, 2005). The identity and amount of each species vary significantly with deriving sources and manufacturing process. A chemical kinetic mechanism that would represent the oxidation of all these species with accompanying chemical reactions is not feasible with current computational capabilities and chemical kinetic knowledge. The compositional complexity of practical jet fuels has created a great challenge for combustion modeling. A promising approach to overcome this difficulty is to first develop a surrogate fuel that can emulate the combustion characteristics of the target jet fuel. In general, a surrogate fuel is defined as a mixture of a limited number of hydrocarbons whose composition can be formulated in order to best match real fuel properties, such as physical properties, chemical properties, or even both (Edwards and Maurice, 2001). The advantage of using a surrogate comes from its relatively small number of components which can be simulated by using the corresponding detailed kinetic mechanism to predict the combustion characteristics of real fuels. The - 3 -
21 selection of surrogate components has typically utilized species of different molecular classes that are found in the real fuel. The issues are which specific components should be used to represent the chemical structure of the real fuels; and how to determine the mixture composition of the surrogate components to best emualte the combustion behaviors of the target real fuel. Moreover, constructing a detailed kinetic model that can enable reliable predictions of many combustion properties of real fuels is also important in the development of surrogate fuels. Recognizing that the accuracy of a surrogate model depends on the comprehensive kinetic sub-model for neat components, thus a better knowledge of combustion characteristics of the reference hydrocarbons is of fundamental and practical importance in the surrogate formulaiton and model development as well as in understanding the combustion behavior of real practical fuels. Over the years, extentive studies have been focused on combustion kinetics of normal alkanes due to their relatively simple structure of straight chain. Little is known for the aromatic species, even they can have a strong effect on the combustion behavior of practical fuels. Speight (2007) has showed that real fuels contain substaintial amounts of aromatics. Aromatic is also important in the formation of polyaromatic hydrocarbons (PAHs) which are generally considered as sooting precursors (Frenklach et al., 1985). In addtion, though alternative fuels are entirely free from aromatics, they have to be blended with certain amount of aromatics to maintain elastomer sealing qualities in order for them to be fully used on aircafts (Edwards, 2010). Therefore, there is an urgent need to refine the current understanding of aromatic combustion characteristics and their coupling performance with other fuel structures present in the transportation fuels
22 Most of the flame studies on liquid hydrocarbons are so far carried out under atmospheric pressure condition. This is because liquid hydrocarbons, especially with large molecular weight, are more difficult in handling compared to light gaseous hydrocarbons. The low vapor pressure of large hydrocarbons imposes a great challenge on experiments in terms of fuel vaporization, preventing fuel condensation and cracking, resulting in a lack of flame data at high pressures. On the other hand, fuels are usually combusted in engines at high pressures. Therefore, to better understand the engine-related combustion, it is necessary to extend current knowledge of flame properties of liquid hydrocarbon fuels to elevated pressures that are close to engine conditions. The high pressure data will not only be valuable to understand the pressure effects on flame properties, but also be useful in the validation of current kinetic models as to see whether they can still be able to capture the flame properties when pressure is changed. 1.2 Combustion Responses Reliable design and optimization of jet engines will rely on a complete understanding of the combustion characteristics of jet fuels. The first step is to obtain the fundamental understanding of various combustion responses in well characterized environments. Two widely used flame parameters are laminar flame speed and extinction stretch rate. These two parameters represent important input in the design of new combustors as well as targets for model development and validation. The laminar flame speed is one of the most important flame parameters of a combustible mixture. It describes the propagation of the one-dimensional, planar, adiabatic, premixed flame in the doubly infinite domain, and embodies the fundamental information on the diffusivity, reactivity, and exothermicity of a given fuel/oxidizer mixture. Therefore, it can be considered as a crucial parameter for - 5 -
23 characterizing fuel combustion. In particular, on a practical level, laminar flame speed affects the fuel burning rate in internal combustion engines and the engine performance and emissions. On a fundamental level, the flame speed is an important target for developing and validating kinetic mechanism. Another important flame property is the extinction limit. The flame extinction is induced by either an incomplete reaction or the non-equidiffusion of heat and mass, and captured by the stretch rate of the flame at extinction. The extinction stretch rate represents a kineticsaffected phenomenon and characterizes the interaction between a characteristic flame/flow time to a chemical reaction time. Flame extinction can be considered as one of the important factors to understanding the complicated turbulent combustion mechanisms in real engines, especially from the viewpoint of flame stabilization. Based on the importance of laminar flame speed and extinction stretch rate, this work is mainly focused on measuring and simulating these two flame properties for various liquid hydrocarbon fuels. 1.3 Scopes and Objectives The primary objective of this dissertation is to experimentally and computationally investigate the premixed flame properties, including laminar flame speed and extinction stretch rate, of practical jet fuels and their surrogates as well as relevant neat hydrocarbon components. The study of practical jet fuels including conventional and alternative fuels can be useful to assess the feasibility of alternative jet fuels in aviation applications. The surrogate fuel study will introduce and examine two surrogate mixtures developed under a methodology that can best emulate combustion behaviors of target real fuels. Another study is focused on the aromatic species, which are far less characterized compared to alkanes. The aromatic study is intended to improve our understanding of combustion kinetics of aromatic species which are - 6 -
24 important components in both real fuels and their surrogate fuels. The last endeavour is made to extend our atmospheric flame propagation study to elevated pressures, which is more relevant to combustion in engine conditions. The results can be used to study the pressure effects on flame propagation of liquid hydrocarbon fuels. Moreover, several kinetic models found in literatures are examined against the present experimental data for different fuels. Further sensitivity and flux analysis are also conducted for aromatic species to identify the key reactions and assess the molecular structure effects on fuel oxidation process. 1.4 Overview of Dissertation In Chapter 2, the experimental setup and procedures will first be introduced, and then followed by the experimental methodologies for determining the laminar flame speed and extinction stretch rate. Chapter 3 describes the computational tools and numerical methods for simulations of laminar flame speed and extinction limit. Chapter 4 presents the experimental data of laminar flame speeds and extinction stretch rates of Jet-A and three alternative jet fuels. The simulation results and sensitivity analysis of Jet-A flames are also presented. Moreover, derived cetane numbers of Jet-A and alternative jet fuels are presented to show the differences in their ignition behaviors. Chapter 5 introduces a surrogate formulation methodology proposed by Dooley et al. (2011, 2012), and presents the experimental data of laminar flame speeds and extinction stretch rates of two surrogate mixtures along with their target fuel Jet-A at atmospheric pressure
25 Chapter 6 presents the experimental and computational data of laminar flame speeds and extinction stretch rates of selected aromatic species, including toluene, n- propylbenzene (n-pb), 1,2,4-trimethylbenzene (1,2,4-TMB), and 1,3,5- trimethylbenzene (1,3,5-TMB). Further sensitivity and flux analysis are also presented to illustrate the structure effects on fuel oxidation process. Chapter 7 presents the experimental and computational data of laminar flame speeds of various liquid hydrocarbon fuels at elevated pressures of 1-3 atm. The effects of preheat temperature and pressure on flame propagation are also presented and discussed. Chapter 8 presents a summary of the results and suggestions for some future work. For completeness, all journal papers that are relevant to this dissertation, and either have been published or under review are listed as below: Chapter 4: Experimental Studies on the Combustion Characteristics of Alternative Jet Fuels, by Hui, X., Kumar, K., Sung, C.J., Edwards, T., and Gardner, D., Fuel, 2012; 98: Laminar Flame Speeds and Extinction Limits of Conventional and Alternative Jet Fuels, by Kumar, K., Sung, C.J., and Hui, X., Fuel, 2011;97(3): Chapter 5: The Experimental Evaluation of a Methodology to Surrogate Fuel Formulation for the Emulation of Gas Phase Combustion Kinetic Phenomena by a Theory of Real Fuel Oxidation, by Dooley, S., Won, S.H., Heyne, J., Farouk, T., Dryer, F.L., Ju, Y., Kumar, K., Hui, X., Sung, C.J., Wang, H., Oehlschlaeger, M.A., Santoro, R.J., Litzinger, T.A., Iyer, - 8 -
26 V., Malewicki, T., and Brezinsky K., Combustion and Flame, 2012;159(4): Chapter 6: Laminar Flame Speeds and Extinction Stretch Rates of Selected Aromatic Hydrocarbons, by Hui, X., Das, A.K., Kumar, K., Sung, C.J., Dooley, S., and Dryer, F.L., Fuel, 2012;97: Chapter 7: Laminar Flame Speeds of Transportation-Relevant Hydrocarbons and Jet Fuels at Elevated Temperatures and Pressures, by Hui, X. and Sung, C.J., submitted
27 Chapter 2 Experimental Specifications 2.1 Experimental Setup This section describes experimental apparatus and procedures that are employed to determine the laminar flame speed and extinction stretch rate of fuel/oxidizer mixtures investigated in this work. Note that most of our atmospheric data presented in Chapters 4-6 are taken in an atmospheric counterflow setup. The details of the atmospheric setup can be found in our previous studies (Hirasawa et al., 2002) (Huang et al., 2004) (Kumar and Sung, 2007). The high pressure data presented in Chapter 7 are taken in a high pressure counterflow setup developed for this thesis work. As shown in Figure 2.1, the high pressure counterflow setup consists of a flow controlling system, fuel vaporization system, high pressure counterflow burner, and a digital particle image velocimetry (DPIV) system. The mixture preparation, flow field measurement, and data processing remain the same for both atmospheric and high pressure counterflow setups, which will be introduced along with the high pressure counterflow burner in the following sections High Pressure Counterflow Burner To be able to measure the flame properties at high pressures, a high pressure counterflow burner was designed, fabricated and tested. The counterflow burner is placed inside a high pressure chamber as shown in Fig. 2.2(a). The high pressure chamber body is made of half-inch thick stainless steel and has a dimension of 50.8 cm in diameter and 33 cm in height. The chamber can be pressured up to 20 atm. A back pressure regulator is employed before the exhaust of the chamber to eliminate the downstream perturbation and ensure a constant pressure inside the chamber. There are
28 four viewing windows perpendicularly mounted to each other on the chamber wall. Each viewing window is sealed with a quartz glass that allows for the access of the laser sheet. Two identical burners are placed vertically inside the chamber and opposed to each other. The burner shown in Fig. 2.2(b) is designed with a high contraction ratio, contoured nozzle to obtain a top hat velocity profile at the exit. The nozzle has an exit diameter of 10 mm; the distance between the two nozzles is kept close to one exit diameter. This nozzle separation distance is sufficiently large, compared to the flame thickness, to prevent significant upstream heat loss from the flame to the burner nozzles over the stretch rate range of interest. In addition, the burner nozzles are provided with an annular co-flow of heated nitrogen which serves to stabilize the flame and also limit the flame interaction with the surroundings. In order to ignite the combustible mixture, a spark igniter made of tungsten wire is employed. The igniter is connected to a high voltage transformer, which can provide the igniter with a peak voltage of 20,000 volts at khz. The spark induced by the high voltage is sufficient enough to ignite the flame at high pressures. After ignition, the igniter can be moved away from flame to avoid any interactions Mixture Preparation The fuel/air mixture is prepared as described below. The liquid fuel is driven and controlled by a high precision syringe pump. The metered liquid fuel is sprayed into a heated prevaporizer chamber with a shrouding nitrogen flow and then vaporized. The shrouding nitrogen flow is heated close to the boiling temperature of liquid hydrocarbon fuel, and the shear force generated by the high speed of the shrouding nitrogen flow can facilitate the atomization process. The atomized fuel droplets then vaporized inside the chamber. Subsequently, the vaporized fuel is mixed with O 2 and N 2 to form the combustible mixture of desired composition. Sonic nozzles are used to
29 regulate the gas flow rates. A bypass valve is employed just before the high pressure burners to vary the flow rate of the fuel/oxidizer mixture through the burners. This arrangement allowed for the variation of the stretch rate without changing the composition and seeding density of the combustible mixture. The entire flow circuit is heated and maintained at an appropriate temperature to prevent condensation of fuel vapor. A nebulizer is employed to generate micron-size silicone droplets as seeding particles to enable the DPIV measurement of the flow flied. The accuracy of the volumetric displacement of the syringe pump is 0.5% of the set point. The temperature controller is able to maintain the gas temperature at desired values with a variation about ±2 K. The variation of chamber pressure is kept less than ±0.03 atm when experiments are conducted. The flow system is calibrated in advance by a wet-test flow meter, and the associated uncertainty is estimated to be within 1% of the total flow rate, which is well within the range of accuracy using this procedure. The synthetic air is prepared by mixing ultra-high purity nitrogen and oxygen. The specifications of the gases and fuels in this work are listed in Table 2.1. Table 2.1 Specifications of gases and fuels Gas/Fuel Purity Vendor Nitrogen 99.99% Airgas Oxygen 99.99% Airgas n-decane 99% Fisher Scientific n-dodecane 99% Fisher Scientific iso-octane >99% Fisher Scientific Toluene 99% Fisher Scientific n-propylbenzene 98% Fisher Scientific 1,2,4-Trimethylbezene 98% Fisher Scientific 1,3,5-Trimethylbezene 99% Fisher Scientific Jet-A POSF Air Force Research Laboratory
30 S-8 POSF Air Force Research Laboratory IPK POSF Air Force Research Laboratory Camelina POSF Air Force Research Laboratory DPIV Measurement Digital Particle Image Velocimetry (DPIV) is a non-intrusive, whole field optical technology used to gain velocity information on so-called seeding particles suspended in a fluid in motion. It is based on the measurement of particle displacement over a known time interval. Therefore, speed can be calculated according to the relationship: Distance, X (m) Speed, V (m s)= Time, t (s) (2.1) Figure 2.3(a) shows the principle of DPIV system. A pulsed Nd:YAG dual head laser is used to illuminate the seeding particles. The laser generates a 200 mj/pulse light beam at 532 nm with 5-10 ns pulse width. The beam is transformed into a light sheet of 0.2 mm thickness through the cylindrical lenses. The light sheet is typically pulsed to produce a stroboscopic effect, freezing the movement of the seeding particles over time. In order to represent flow velocity, the seeding particles must be small enough to track the flow accurately and yet big enough to scatter sufficient light for the camera to be able to detect them. In the present study, the flow is seeded by 1-2 µm diameter droplets with a viscosity of 50 centistokes produced by the nebulizer made from Sunrise Medical HHG Inc. Since the boiling point of the silicone oil is above 570 K, the seeding droplets cannot survive through the flame as shown in Fig. 2.3(b). The positions of the illuminated seeding particles are captured by a CCD camera with a resolution of pixels, which is positioned perpendicular to the light
31 sheet. Particles then appear as light specks on a dark background on each camera frame. Further, the light source and the camera are synchronized so particles illuminated at the instant of light pulse number 1 are captured on camera frame 1, and particles from light pulse number 2 are on frame 2. For PIV data evaluation, the camera image is divided into subregions of pixels for interrogation. Each of these regions is correlated (from frame 1 to frame 2) with a 50 percent overlap to produce an average particle displacement vector. Doing this for all the interrogation areas generates a vector map. Transforming camera displacement to physical dimensions and dividing by the time between the two frames, displacement vectors are converted into a map of raw velocity vectors as shown in the bottom images in Fig. 2.3(b). Validation algorithms can then be applied to this raw vector map to improve the quality of the PIV results. Following the study of Wernet (2000), the full-scale accuracy in the velocity measurement of the present DPIV system is determined by: Full-scale accuracy = [(σ T) + (σ D) ], (2.2) where σ is the discriminative minimum period between pulses, T is the period between pulses, σ is the discriminative minimum displacement, and D is the maximum displacement determined by the 1 4 rule. D is 8 and 12 pixels for the subregion size of pixels without and with 50% subregion overlapping, respectively. For particular images spanning 1-2 pixels, the discriminative minimum σ is 0.1. The timing error σ T is typically negligible when using Nd:YAG laser. Consequently, the full-scale accuracy in velocity measurement is equal to σ D, which respectively leads to =1.25% and =0.83% in radial and axial directions for the present setup
32 2.2 Laminar Flame Speed Determination Laminar flame speed, S, is a global fundamental and practical flame property of a fuel/oxidizer mixture. It is defined as the velocity of a steady, one- dimensional, laminar propagation of a planar, adiabatic combustion wave into a uniform mixture of the fuel/oxidizer. Because of the fundamental significance of the laminar flame speed in premixed flames, a considerable amount of efforts has been expended toward its determination. A major difficulty in its determination is that a planar, stationary, and adiabatic flame rarely can be achieved. Several flame speed measuring techniques have been developed to address the issue. These techniques have employed either stationary burner flames held by an upstream flow, or propagating flames in open and closed chambers. In the following sections we will particularly discuss the counterflow twin-flame technique that is used in this work for the determination of laminar flame speed Counterflow Twin-Flame Configuration In the counterflow setup, the premixed combustible mixtures are impinged from two convergent nozzles opposed to each other. Upon ignition two symmetrical flat flames are situated on both sides of the stagnation surface as shown in picture in Fig Figure 2.4 shows the schematic of the counterflow twin-flame configuration and axial velocity along the centreline. It can be seen that as the flow approaches the stagnation plane, but before reaching the main preheat region, the axial velocity decreases linearly as V = Kx, where x is the distance to stagnation plane, in accordance with the characteristic of potential stagnation flow, where K = dv dx. However, as the flow enters the preheat region, intense heating and thereby thermal expansion reverse
33 the decreasing trend and cause the velocity to increase. Eventually in the post flame zone, the velocity decreases again as it approaches the stagnation plane. For such an axial velocity profile, V can be identified and defined as a reference flame speed, S, at the upstream boundary of the preheat zone where the flame is stabilized, and the maximum axial velocity V can be identified at the downstream boundary of the reaction zone. These values can be considered to be obtained under adiabatic conditions because the upstream heat loss for the nozzle-generated flow is small while the downstream heat loss is also small due to symmetry. It has also been found that radiative heat loss is negligible when the flame is well within flammability limits (Vagelopoulos et al., 1994). The stagnation flame is also subjected to flame stretch effects, which is characterized by the negative of the axial velocity gradient, K. According to the theory for an axisymmetric stagnation flow, the local stretch rate at reference location as shown in Fig. 2.5(a) can be determined by: K = dv dx = 2 du dr (2.3) where V is the axial velocity and U is the radial velocity. However, in experiments, the axial velocity profile is more like plug flow; the velocity gradient continuously varies from nozzle exit towards the reference location. Thus, there is some ambiguity in defining the representative stretch rate based on the axial velocity profile. To avoid this ambiguity, the flame stretch rate is defined by using the radial velocity profile at the reference point, which can be seen to be linear in Fig. 2.5(b). It has been suggested that the radial velocity gradient yields a more precise estimate of stretch rate (Kumar, 2007). Then the flame stretch rate, K, is equal to twice the radial velocity gradient
34 2.2.2 Linear and Nonlinear Extrapolations The laminar flame speed, S, can be determined by extrapolating the reference flame speed, S, to zero stretch rate by either linear or nonlinear extrapolation. Figure 2.6(a) plots the reference flame speed versus stretch rate for n-pb/air mixtures at an unburned mixture temperature of T =400 K, pressure of P=1 atm, and equivalence ratios of =0.7, 1.0, and 1.4. The equivalence ratio is defined as ϕ = ( ) ( ), where F/O is the mass ratio of fuel to oxidizer in the mixture; the subscript st designates the stoichiometric state. Thus, ϕ < 1, = 1, and > 1 respectively correspond to fuel-lean, stoichiometric, and fuel-rich combustion. From the Fig. 2.6(a), it can be seen S varies approximately linearly with stretch rate, K, at all fuel-lean, stoichiometric, and fuel rich conditions. Therefore, laminar flame speed, S, can be identified by extrapolating S to zero K, since both heat loss and flow non-uniformity effects are eliminated. Mathematically, the linear relation between S and K can be given by Clavin (1985) as below: S = S [1 + μk S ] (2.4) where μ is of the order of the flame thickness and known as the Markstein length. The coefficient μ depends on thermal expansion and on the Lewis number, Le. In particular, μ changes sign as Le crosses a critical value near unity, so that S increases or decreases for mixtures with Le less than or greater than unity, respectively. It can be confirmed in Fig. 2.6 by the positive and negative slopes for the rich n-pb/air mixture ( =1.4, Le=0.95) and lean n-pb/air mixture ( =0.7, Le=2.96), respectively
35 The linear relation of Eqn. (2.4) is based on the assumption that the characteristic length associated with diffusion L is much smaller than the characteristic scale of the fluid flow L. Then combustion takes place within a thin but finite layer on the order of δ = L L. When δ 0, the whole flame shrinks to a single surface that coincides with the reaction sheet. The flow field on either side of the flame front is determined by the inviscid and incompressible equations of motion because there diffusion and chemical reaction are negligible. The inner structure of the flame yields the appropriate conditions for an equation similar to Eqn. (2.4). In the linear relation formulation, the flame speed has been determined as local velocity of the cold gas just ahead of the flame front. However, for the evaluation of the flame speed at different reference position within the flame zone, Eqn. (2.4) should be corrected to account for the O(δ) displacement. This correction, which becomes negligible as δ 0, cannot be ignored when determining the laminar flame speed of stretched flames because the effect of stretch appears exactly in the O(δ) term (Tien and Matalon, 1991). As discussed earlier, we have used the minimum velocity as the reference flame speed to determine laminar flame speed. In this situation, Tien and Matalon (1991) suggested that a linear extrapolation, used to determine the flame speed at zero strech rate, would lead to an over-prediction of S. A higher order fitting of experimental data of S versus Karlovitz number Ka would yield to a more accurate S as shown below: S = S {1 (μ 1)Ka + Kaln[(σ 1) Ka]} (2.5) where Ka is defined as Ka = Kα (S ), α is the thermal diffusivity of the unburned mixture, σ is the thermal expansion parameter represented by the density ratio of unburned to burned mixtures, and μ is a non-dimensional parameter
36 depending on the physicohemical properties of the unburned mixture and can be estimated along with S through the fitting. The Ka can be interpreted by the ratio of flame thickness α S to the charateristic length of the flow S K, it has the same magnitude as O(δ). Therefore, when Ka number is small enough, the difference between linear and nonlinear extrapolation is also small. Vagelopoulos et al. (1994) and Chao et al. (1997) have demonstrated that when Karlovitz numbers are kept to the order of O(0.1), the accuracy of linear extrapolation is improved and the over-prediction can be reduced to be within the experimental uncertainty. Figure 2.6(b) plots the reference flame speed versus Kalovotiz number of n-pb/air mixture at the same conditons in Fig. 2.6(a). It can be seen that the maximum difference between linear and nonlinear extrapolation is about 4 cm/s for n-pb/air mxture at =1.0. In the present work, we have attempted to use the nonlinear extrapolation method based on the theoretical analysis of Tien and Matalon (1991) to report the laminar flame speeds for most of the fuels investigated. However, the thermal and transport properties required for such nonlinear extrapolation are not available for complex blended fuels; in such cases, the linear extrapolation has been used. Nevertheless, the extrapolation method will be specified when laminar flame speed data are presented. Finally, the standard error value associated with the extrapolation procedure to deduce the laminar flame speed is shown as an error bar when plotting the reported data. 2.3 Extinction Stretch Rate Determination The extinction stretch rate K is also determined in the counterflow twin-flame setup by using PIV system. Since K cannot be directly measured, it requires a nearextinction twin-flame to be established first, and then the flow rates through the
37 burners are gradually increased until an abrupt flame blow-off is observed. Just prior to the flame extinction, the stretch rate K at reference point ahead of the flame is measured. Experiments and simulations both show that the change in K due to the slight change in the flow rate is inconsequential and the stretch rate K just prior to flame extinction can be identified as extinction stretch rate K. Similar to flame speed measurement, the extinction stretch rate K is based on twice the radial velocity gradient at the reference flame speed location. Typically, a sequence of 32 image pairs is captured just before the extinction. The image pairs are then processed to determine the corresponding extinction stretch rate. Therefore, the extinction stretch rate value reported herein is the averages from 32 or more velocity maps obtained from repeated runs
38 Figure 2.1 Schematic of the flow control system, high pressure counterflow burner setup, and DPIV system
39 (a) Flange Fuel inlet Cooling compartment Coflow inlet Nozzle exit (b) Figure 2.2 (a) High pressure chamber; (b) counterflow burner
40 (a) (b) Figure 2.3 (a) Schematic of PIV measurement principle (Dantec, 2006); (b) images and velocity maps with and without flames
41 Axial Velocity, V (cm/s) (a) 80 Stagnation plane K=-dV/dx V max 20 V min Distance to Stagnation Plane x (cm) 0 (b) Figure 2.4 (a) Schematic of the counterflow twin flame configuration; (b) axial velocity profile along central line (simulated)
42 Axial Velocity (cm/s) Radial Velocity (cm/s) Reference Flame Speed Reference Location Distance from Nozzle Exit (mm) (a) Radial Distance from Centerline (mm) (b) Figure 2.5 PIV measured velocity profile, (a) axial velocity; (b) radial velocity
43 Reference Flame Speed (cm/s) n-pb/air Mixtures, Tu=400 K and P=1 atm 100 = = =0.7 Experimental data 20 Linear extrapolation Nonlinear extrapolation Stretch Rate, K (s-1) Reference Flame Speed (cm/s) (a) n-pb/air Mixtures, Tu=400 K and P=1 atm 100 = = =0.7 Experimental data 20 Linear extrapolation Nonlinear extrapolation Karlovitz Number, Ka (b) Figure 2.6 Reference flame speed of n-pb/air mixtures at an unburned mixture temperature of 𝑇 =400 K and equivalence ratios of =0.7, 1.0, and 1.4, along with the demonstration of linear (solid lines) and nonlinear (dashed lines) extrapolations, (a) reference flame speed versus stretch rate; (b) reference flame speed versus Karlovitz number
44 Chapter 3 Computational Specifications 3.1 Flame Propagation The laminar flame speeds are simulated by using a freely-propagating, steady, adiabatic flame in Sandia PREMIX code (Kee et al., 1985) in conjunction with CHEMKIN (Kee et al., 1989) and TRANSPORT packages (Kee et al., 1998). The simulations used windward differencing on the convective term, and considered the thermal diffusion of H and H 2. The algorithm employed automated coarse-to-fine grid refinement as a mean to enhance the convergence properties of the steady-state approach and as a mean to provide optimal mesh placement. Assuming one-dimensional flow with uniform inlet conditions, the governing equations can be given by: Continuity: M = ρua (3.1) Energy: M dt dx 1 c d dt (λa dx dx ) + A c ρy V c dt dx + A c ω h W = 0 (3.2) Species: M dy dx + d dx (ρay V ) Aω W = 0 k = K (3.3) Equation of State:
45 ρ = PW RT (3.4) In Eqns. (3.1)-(3.4), x denotes the spatial coordinates; M the mass flow rate; T the temperature; Y the mass fraction of the kth species; P the pressure; u the velocity of the fluid mixture; ρ the mass density; K is the total number of the species; W the molecular weight of the kth species; W the mean molecular weight of the mixture; R the universal constant; λ the thermal conductivity of the mixture; c the constant pressure heat capacity of the mixture; c the constant pressure heat capacity of the kth species; ω the molar rate of production by chemical reaction of the kth species per unit volume; h the specific enthalpy of the kth species; V the diffusion velocity of the kth species; and A the cross-section area of stream tube encompassing the flame normalized by the burner area. The net chemical production rate ω of each species results from a competition between all the chemical reactions involving that species, and each reaction proceeds according to the law of mass action and the forward coefficients are in the modified Arrhenius form: k = BT exp ( E RT ) (3.5) where k is the forward reaction coefficient; B is the frequency factor; T is the temperature; β is the temperature exponent; E is the activation energy. The details of the chemical reaction equations and the thermochemical properties can be found in (Kee et al., 1989), which discussed the evaluation of these expressions. When the problem is posed on a finite domain [0, L], the boundary conditions can be given by:
46 At the cold boundary, x = 0: T T = 0 (3.6) ε Y ( ρay V M ) = 0 (3.7) where T is the specified unburned mixture temperature and ε is the inlet reactant fraction of the kth species. At the hot boundary, x = L: dt dx = 0 (3.8) dy dx = 0 (3.9) In the flame propagation flames, M is the eigenvalue and must be determined as part of solution. Therefore, an additional constraint is required, which can be obtained by fixing the temperature at one point, T(x ) T = 0 (3.10) This internal condition of Eqn. (3.10) completes the problem and allows for the solution of the flame speed eigenvalue M. The constraint temperature and point should be selected in such way as to insure that the temperature gradients nearly vanish at the cold boundary
47 3.2 Extinction Limit The extinction stretch rates are simulated using the opposed-flow code (Kee et al., 1988) in conjunction with CHEMKIN (Kee et al., 1989) and TRANSPORT (Kee et al., 1998) packages. The opposed-flow code is modified by using the one-point temperature controlling method of Nishioka et al. (1996) to generate the flame response curve. The turning point of the flame response curve defines the extinction limit. At the turning point, the computed maximum axial velocity gradient ahead of the flame is used to determine the extinction stretch rate. Consistent with the experimental determination, in the opposed flame modeling the local stretch rate based on the axial velocity gradient is equal to twice the radial velocity gradient. Figure 3.1(a) plots the computed profiles of axial velocity and temperature for stretched premixed flames at two different stretch rates, and is used to illustrate the definition of stretch rate used in the computations. Figure 3.1(b) further shows the typical flame response curve by plotting maximum flame temperature to stretch rate variation. Note again, that the point of vertical tangency to the curve shown in Fig. 3.1(b) is defined as the extinction stretch rate. A brief summary of the governing equations that simulate counterflow premixed flames is shown below. In the opposed-flow model, a steady state solution is computed for axisymmetric premixed flames between two opposing nozzles. Due to the symmetric structure, the three-dimensional flow can be reduced mathematically to quasi-one dimension by assuming that radial velocity varies linearly in the radial direction, which leads to a simplification in which the fluid properties are functions of the axial distance only. At steady state, conservation of mass in cylindrical coordinates is
48 (rρu) + (rρv) = 0 (3.11) x r where u and v are the axial and radial velocity components, and ρ is the mass density. Recognizing the v r and other variables should be functions of x only, two functions G(x) and U(x) can be defined as: G(x) = (ρv) r U(x) = 1 2 ρu then the continuity Eqn.(3.11) reduces to G(x) = du(x) dx (3.12) The radial momentum equation is satisfied by the eigenvalue H = 1 r p = constant (3.13) r Radial momentum equation is H = d dx (2UG ρ ) 3 ρ G d dx [μ d dx (G ρ )] = 0 (3.14) Energy and species equations are ρu dt dx 1 c d dt (λ dx dx ) + ρ c c Y V dt dx + 1 c h ω W = 0 (3.15) ρu dy dx + d dx (ρy V ) ω W = 0 k = 1 2 K (3.16)
49 where T is the temperature, c the mixture specific heat, μ is the dynamic viscosity and λ the mixture thermal conductivity. The quantities Y, V, c, ω, W, and h are the mass fraction, diffusion velocity, specific heat, molar production rate, molecular weight and specific enthalpy for the species k, respectively. Boundary conditions are given by: At nozzle outlet, x = L: U( L) = ρ u 2 ; G( L) = 0; T( L) = T ; Y ( L) = Y (3.17) At stagnation plane, x = 0: U(0) = 0; dg dy = 0; dx dx dt = 0; = 0; (3.18) dx The differential Eqns. (3.12) through (3.16) and boundary conditions Eqn. (3.17) and (3.18) form a boundary value problem for the dependent variables of U, G, H, T, Y. The gas-phase kinetics subroutine library provides the reaction rates and thermodynamic properties, while the transport package evaluates the transport properties for these equations. 3.3 Mixture-averaged and Multicomponent Transport Properties Both the PREMIX and opposed-flow codes allow for the use of the mixture-averaged or multicomponent formulations to evaluate molecular transport properties. Since the multicomponent diffusion coefficients, thermal conductivities, and thermal diffusion coefficients are computed from the solution of equations of the system, it generally leads to more accurate transport properties and is preferable for extinction stretch rate calculation. However, the multicomponent formulation is significantly more
50 computationally expensive and results of premixed hydrocarbon flames by using multicomponent formulation are expected to be within 5% of those by using mixtureaveraged formulation (Ji et al., 2010). Figure 3.2 shows the differences between these two transport formulations in laminar flame speed and extinction stretch rate calculations for 1,2,4-TMB flames. It can be seen that the differences are mostly less than 3% in both laminar flame speed and extinction stretch rate simulations. Therefore, all the computed results presented in this work are performed by using the mixture-averaged formulation for the ease of computation. In the mixture-averaged formula, the diffusion velocity V is composed of three parts: V = V + V + V (3.19) V is the ordinary diffusion velocity and is given in the Curtiss-Hirschfelder (Hirschfelder et al., 1954) approximation by: V = D 1 X dx dx (3.20) where X is the mole fraction, and the mixture-averaged diffusion coefficient D is given explicitly in terms of the binary diffusion coefficients D D = 1 Y X D (3.21) V is thermal diffusion velocity for the low molecular weight species H, H 2, and He, and is determined by:
51 V = D Θ X 1 dt T dx (3.22) where Θ is the thermal diffusion ratio. The sign of Θ makes the lower (higher) molecular weight species diffuse from low (high) to high (low) temperature regions. The correction velocity V is included to insure that the mass fractions sum to unity or equivalently, thus the formation of correction velocity is recommended by Coffee and Heimerl (1983). Y V = 0 (3.23) In the case of viscosity, the mixture-averaged formulation uses the semi-empirical formula due to Wilke (1950) and modified by Bird et al. (1960) The Wilke formula for mixture viscosity is given by μ = X μ X Φ (3.24) where Φ = 1 W (1 + ) [1 + ( μ 8 W μ ) ( W ) ] W (3.25) For the thermal conductivity of the mixture, a formula proposed by Mathur et al. (1967) is used λ = 1 2 ( X λ + 1 X λ ) (3.26)
52 Maximum Flame Temperature, T max (K) Axial Velocity, u (cm/s) Temperature (K) Axial Velocity and Temperature Profiles K 2 = (du/dx) max =1081 s K 1 = (du/dx) max =549 s N 2 /(N 2 +O 2 )=0.84 Aachen Kerosene Surrogate Distance from Stagnation Plane, x (cm) (a) 1850 Flame Response Curve 1800 ( K 1, T max,1 ) Strongly Burning N 2 /(N 2 +O 2 )=0.84 Aachen Kerosene Surrogate ( K 2, T max,2 ) Near Extinction Stretch Rate (s -1 ) (b) Figure 3.1 (a) Computed axial velocity and temperature profiles and (b) computed flame response curve
53 Ratio of Value mult /Value mix-avg ,2,4-TMB Flames, T u =400 K and P=1 atm Laminar Flame Speed Extinction Stretch Rate Equivalence Ratio, Figure 3.2 Differences between mixture-averaged formulation and multicomponent formulation in laminar flame speed and extinction limit simulations for 1,24-TMB flames at T =400 K and P=1 atm
54 Chapter 4 Flame Propagation and Extinction of Jet-A and Alternative Jet Fuels 4.1 Background Because of limited petroleum availability, the alternative and renewable energy resources become increasingly important for several reasons such as costs, greenhouse emissions, reduction of fuel import dependency, and security of supply. Sustainability in energy supplies requires new concepts as well as improvements in overall efficiency and fuel flexibility. In aviation sector, the only worldwide available jet fuel is nearly exclusively based on kerosene. The primary fuel for commercial aircraft of the United States is Jet-A, which is complex mixture composed of higher order hydrocarbons, including alkanes, cycloalkanes, and aromatic molecules. While the dependence of the aviation sector on fossil fuels is expected to continue for the foreseeable future, concerns about rising fuel price, energy supply, energy security, climate change, and aviation emissions call for a fresh look at the use of the alternative jet fuels. An aviation-industry-wide-effort to develop alternative jet fuels is being coordinated by the Commercial Aviation Alternative Fuels Initiative (CAAFI). The Industry has agreed that any alternative jet fuels developed should be drop-in fuels, requiring no changes in aircraft or infrastructure equipment. Based on the feedstock, alternative jet fuels are often divided into two categories: synthetic fuels and renewable or bio-jet fuels, although this terminology has limitations. Synthetic fuels are derived from fossil feedstocks such as coal and natural gas. The most common synthetic fuel process is based on the Fisher-Tropsch (F-T) process. Raw material is first gasified to produce a mixture of carbon monoxide and hydrogen known as synthesis gas. The synthesis gas is then converted to liquid
55 hydrocarbons by using the F-T process. The Fischer-Tropsch process is often termed indirect liquefaction when referring to coal-derived fuels, and fuels are often referred to as CTL (coal-to-liquids) or GTL (natural gas-to-liquids). One of the difficulties of the synthetic fuel terminology comes when the F-T process produces fuel from biomass (BTL) or coal/biomass mixtures (CBTL) thus, not all F-T fuels are synthetic fuels in the sense of being fossil-derived. Jet fuel can also be produced by direct liquefaction of coal (Edwards et al., 2010). Thus one can also speak of non-f-t synthetic jet fuels. Figure 4.1 shows the molecular distributions and compositions of five typical F-T synthetic jet fuels including Sasol IPK (Iso-Paraffinic Kerosene), S-8, Shell GTL, and two Sasol GTLs (Moses, 2008). It can be seen that the synthetic jet fuels consist almost entirely of n-paraffins and iso-paraffins, although relatively small amount of cyclo-paraffins are present in some fuels. These alternative jet fuels contain no or very trace amount of aromatics and olefins. The F-T synthetic fuels are thus commonly termed as Synthetic Paraffinic Kerosene (SPK). Renewable or bio-jet fuels are produced from various types of biomass (lignocellulosic/woody, biomass, sugars/starches, and plant oils/animal fats) by a variety of processes. The most-studied aviation biofuels at this point are produced from plant oil sources (algae, camelina, jatropha, etc.) and animal fats (tallow) through hydroprocessing, thus are termed as Hydroprocessed Renewable Jet (HRJ) or Hydroprocessed Esters and Fatty Acids (HEFA) fuels. The oil from such sources is hydrotreated to remove the chemicallybound oxygen and to produce proper molecular weight components for jet fuels. The gas chromatographic analysis of HRJ fuels demonstrates that they have similar molecular distribution as F-T SPK fuels (Rahmes et al., 2009), as shown in Fig
56 4.1Figure 4.1(b). This similarity of HRJ and SPK fuels which does not depend on feedstock is expected to increase the fuel choice and flexibility. Alternative aviation fuels have been undergoing extensive development in recent years. Since the mid-1950s, Sasol in South Africa has been producing synthetic hydrocarbon fuels using a high temperature Fischer-Tropsch process. In 1999, approval was granted to Sasol (only) for blending up to 50% of Sasol IPK into conventional petroleum-derived jet fuels in South Africa. In 2009, the efforts of US Air Force and CAAFI to develop a generic F-T SPK specification culminated in the approval of ASTM D7566, the first alternative aviation fuel specification. Annex 1 of this specification described the requirements for F-T SPK jet fuels to be approved for addition to petroleum jet fuels up to 50% by volume. Further annexes are anticipated for other types of alternative fuels. In fact, the approval in July 2011 of Annex 2 for HRJ/HEFA can be traced back to the DARPA Bio-jet program (initiated in 2007), Boeing/UOP flight demonstrations in December 2008/January 2009 (Kinder and Rahmes, 2009), and significant US Air Force and Navy programs initiated in 2009 (Corporan et al., 2010) (Air Force Energy Plan, 2010). Further annexes are also anticipated for fuels produced from biomass through pyrolysis/upgrading, fermentation to alcohols followed by dehydration/oligomerization, and direct fermentation of biomass to hydrocarbons. Since some of these processes produce fuels with compositions dramatically different from the fuels shown in Fig. 4.1, this leads to the current interest in continuous improvement of the fuel certification process. The certification process for these alternative jet fuels consists of extensive tests including initial physical property and composition tests, rig/component tests, engine tests, and flight tests. This process is codified in ASTM D4054, but the approval process is under continuous refinement to reduce cost and improve the quality of the
57 data obtained. Fuel performance data is shared with manufacturers to improve their processes in the early stages of the fuel evaluation. There are significant cost benefits that can be obtained by developing a more streamlined combustion testing program that includes fundamental combustion research (Edwards et al., 2010). This fundamental combustion data could enable fuel manufacturers to improve their processes, as well as screening out unacceptable fuels prior to expensive engine tests. The premise for approving alternative jet fuels is that they must be fully interchangeable with current fuels in performance and handling without compromising flight safety. However, due to varying feedstock and manufacturing processes, alternative jet fuels can vary significantly in composition as compared to conventional jet fuels. Thus, substitution of conventional jet fuels with alternative jet fuels requires comprehensive knowledge of the combustion characteristics of alternative jet fuels, including ignition response, flame propagation, and extinction limits. These fundamental combustion properties can be very sensitive to the fuel composition and structure, and can greatly affect the engine performance in terms of ignition, altitude relight, and blowout limits. Therefore, an understanding of the similarities and differences in the fundamental combustion properties of conventional and alternative jet fuels is quite necessary. Some efforts have already been devoted to the fundamental combustion studies of alternative jet fuels. Kumar and Sung (2010a) and Kumar et al. (2010) have compared the autoignition delays, laminar flame speeds, and extinction limits of Jet-A and synthetic S-8. Allen et al. (2011) have compared the autoignition delay times of JP-8 and camelina-based HRJ fuel. Mze-Ahmed et al. (2010) studied the oxidation kinetics of a synthetic jet fuel in a jet stirred reactor. They proposed a detailed kinetic reaction mechanism for synthetic jet fuels from low to high temperature range. Naik et al
58 (2011) developed a detailed kinetic mechanism for alternative jet fuel surrogates. Kick et al. (2011) reported laminar flame speed data for two synthetic jet fuels and proposed a detailed kinetic model. Despite these previous efforts, there is still a lack of experimental combustion data for alternative jet fuels, especially for those that have been developed lately and are of US Air Force and commercial aviation interest. In the present work, we mainly focused on the flame propagation and extinction limit of Jet-A, two SPK fuels including Syntroleum S-8(labelled as POSF 4734) and Sasol IPK (POSF 5642), and one HRJ fuel of UOP camelina (POSF 6152). In addition, we have also provided the Derived Cetane Number (DCN) for these fuels to characterize their ignition behaviors. Some of the physical properties of these jet fuels are shown in Table 4.1. It should be noted that the molecular weights of the alternative jet fuels are derived from their boiling points and specific gravity using Maxwell s empirical formula on hydrocarbons (Maxwell, 1975). The molecular formula for the POSF 4658 Jet-A is reported in the study of Dooley et al. (2010) and is obtained by using a CHN analyzer with the ASTM D5291 method. More details about the thermophysical properties of the tested alternative jet fuels can be found in the studies (Moses, 2008, 2009) (Rahmes et al., 2009). The objective of this work is to provide fundamental combustion data for alternative jet fuels and Jet-A over a wide range of conditions. The data can be utilized to assess the feasibility of alternative fuels in aviation applications as compared to conventional jet fuels and will be helpful in developing a streamlined combustion testing program to reduce expensive rig and engine testing. Moreover, these fundamental data can also be used to develop and validate combustion models to enable quantitative emission and performance predictions in engine design
59 Table 4.1 Physical properties of Jet-A and alternative jet fuels Jet-A Syntroleum S-8 Sasol IPK UOP Camelina POSF Net heat of combustion MJ/kg Density at15 o C kg/l Viscosity at -20 o C mm 2 /s Flash point o C Freezing point o C <-78 <-77 Mean boiling point o C API gravity at 60 o F H/C ratio by mole Molecular weight g/mol Molecular formula C H C H C H C H Kinetic Models Two kinetic models are used in the simulation of laminar flame speed and extinction stretch rate. Jet-A flames are simulated by using a semi-detailed kinetic mechanism of Honnet et al. (2009). The mechanism is referred to Aachen surrogate (80% n-decane, 20% of 1,2,4-TMB by mass) for Jet-A, which has 122 species and 900 reactions. The laminar flame speeds of S-8/air mixtures are simulated by using the surrogate (ndecane/n-dodecane/iso-octane=25/43/32 by mole) and mechanism proposed by Naik et al. (2011). This mechanism has 564 species and 3556 reactions. Due to its relatively large size, the simulation of extinction stretch rate for S-8 flames is not feasible at this time. 4.3 Results Figure 4.2 shows the dependence of reference flame speed on local stretch rate for Jet- A/air, S-8/air, IPK/air, and camelina/air mixtures at an unburned mixture temperature of T u =400 K and equivalence ratios of =0.8, 1.0, and 1.2, as well as demonstrates the
60 linear extrapolation to zero stretch rate for the determination of laminar flame speed. It can be seen that the response of reference flame speed to stretch rate variation for the tested fuels are quite similar at all the conditions shown in Fig At lean and stoichiometric conditions, most of the reference flame speed data overlap, while reference flame speeds of S-8 are slightly higher than those of IPK and camelina at rich condition. We further note that the variation in slopes of the reference flame speed stretch rate dependence at different equivalence ratios is caused by the nonunity Lewis number effect, as discussed in (Law, 1989). Since the thermal and transport properties of Jet-A and alternative fuels required for nonlinear extrapolation are not available, therefore the laminar flame speeds reported in this chapter are extrapolated by using linear extrapolation method. Figure 4.3 compares the atmospheric laminar flame speeds of Jet-A/air, S-8/air, IPK/air, and camelina/air mixtures at T u =400 K and 470 K. It can be seen that with T u increased by 70 K, all the laminar flame speeds increase by an average about 30%. Within the experimental uncertainty, Jet-A has the similar laminar flame speed as S-8, IPK, and camelina. This is because that laminar flame speed has a strong dependence on the adiabatic flame temperature, which is directly controlled by the heat of combustion. As shown in Table 4.1, Jet-A and all the alternative jet fuels tested have the similar heat of combustion, resulting in their similar laminar flame speeds. The other alternative jet fuels, while not investigated for laminar flame speed measurements, are expected to have similar laminar flame speed values as well. Based on the flame speed results, it can be concluded that the laminar flame speeds of the alternative jet fuels are mainly dominated by the flame temperature rather than their fuel compositions
61 Figure 4.4 plots the experimental measured and computed values of laminar flame speeds of Jet-A/air mixtures as a function of equivalence ratio at preheat temperatures of 400 K and 470 K. It can be seen that the mechanism of Honnet et al. (2009) underpredicts the laminar flame speeds of lean Jet-A/air mixtures, and over-predicts the laminar flame speeds of Jet-A flames at stoichiometric and rich conditions except for =1.4. The over-predictions are seen to be more prominent at higher preheat temperature of 470 K than 400 K. Overall, the mechanism has an average deviation of 8% with the experimental data. Figure 4.5 plots the experimental measured and computed values of laminar flame speeds of S-8/air mixtures as a function of equivalence ratio at preheat temperatures of 400 and 470 K. It can be seen that the predictions by the mechanism of Naik et al. (2011) agree very well with the experimental data within an average deviation of 5%, some under-predictions can be observed for rich S-8/air mixtures, especially at preheat temperature of 400 K. Figure 4.6 compares the laminar flame speeds of Jet-A/air, S-8/air, and n- decane/air mixtures as a function of equivalence ratio at preheat temperatures of 400 and 470 K. As discussed earlier, Jet-A and S-8 have similar flame speeds, while n- decane has a discernible higher flame speeds than Jet-A and S-8 at most of the equivalence ratios. Based on the hydrocarbon class distribution shown in Fig. 4.1(a), S-8 is mostly composed of iso-paraffins, which usually have lower flame speeds than the normal paraffins (Huang et al., 2004) (Kumar and Sung, 2010b) (Davis and Law, 1998). As for Jet-A, there is generally 15-25% of aromatics present in the composition. It has been shown by earlier study of Davis et al. (1996) that pure aromatic fuels exhibit a reduced laminar flame speed as comapred to normal alkanes
62 Therefore, these reasons are responsible for the reduced lamianr flame speeds of S-8 and Jet-A when compared to n-decane. Figure 4.7 depicts the experimentally determined extinction stretch rates of Jet- A/oxidizer, S-8/oxidizer, IPK/oxidizer, and camelina/oxidizer mixtures as function of equivalence ratio at atmospheric pressure and unburned mixture temperatures of T u =400 K and 470 K, respectively. Note that the oxidizer in this extinction limit study is composed of 86% N 2 and 14% O 2 (by mole). It can be seen from Fig. 4.7 that though the extinction stretch rates of all the fuels are close to one another, there still exists some differences, and the differences become more discriminative under fuel rich conditions. Among the tested fuels, it can be seen that HRJ-camelina flames have the highest resistance to extinction, followed by S-8 and IPK flames which have similar resistances that are slightly lower than HRJ-camelina, and Jet-A flame has the lowest resistance which is about 8% lower than HRJ-camelina. This lower resistance of Jet-A can be caused by the difference in composition between Jet-A and alternative jet fuels, since Jet-A contains about 20% aromatics, while alternative jet fuels are aromatics free. It has been shown by Kumar and Sung (2010b) and Won et al. (2010, 2011) that aromatics are generally less resistant to extinction than alkanes due to their slow disintegration of the aromatic ring and hence lower reactivity for aromatics as compared to the oxidation of aliphatics. Compared to laminar flame speed results, extinction results show a greater sensitivity to the fuel composition. It can also be seen that the peak of extinction stretch rate for all the flames is at equivalence ratio of =1.5, which is much richer than the value where laminar flame speed peaks. This is due to the combined effects of sub-unity Lewis number for rich jet fuel flames and positive stretch in the counterflow configuration (Kumar and Sung, 2007) (Law, 1989)
63 Figure 4.8(a) plots computed maximum flame temperature response to stretch rate variations of Aachen surrogate at a preheat temperature of 400 K. As expected, for the cases shown, the maximum flame temperature in the upper stable branch decreases with increasing stretch rate when approaching extinction. Again, the stretch rate at the turning point of a given response curve represents the corresponding extinction limit. It is of interest to note that the computed maximum flame temperatures at the extinction turning point lie in a relatively narrow range of K, similar to the results of an earlier study on n-decane and n-dodecane (Kumar and Sung, 2007). The computed extinction stretch rates of Aachen surrogate are compared to those of Jet-A in Fig. 4.8(b). It should be noted that the simulation and experiments shown in Fig. 4.8 are conducted in diluted air of 84% N 2 and 16% O 2. It can be seen that the Aachen surrogate significantly over-predicts the extinction stretch rates of Jet-A by a maximum deviation about 50% at ϕ=1.4. However, the qualitative features such as the shape of the curve and the location of the peak at fuel rich mixtures are captured by the computations using this mechanism. It is further noted that the over-prediction of extinction stretch rate could be either due to the deficiencies of the combustion chemistry, transport/thermodynamic property uncertainties, quasi-one-dimensional nature of the counterflow flame modeling, or a combination of some or all of the aforementioned factors. Clearly, further studies are required to reliably attribute the observed discrepancies to each of these factors. A comparison of the extinction conditions for real jet fuels and typical pure alkane components is shown in Fig It can be seen that the relative extinction stretch rates of both Jet-A and S-8 are lower compared to typical single component of jet fuel surrogates such as n-decane and n-dodecane. The general nature of the dependence of the extinction stretch rate on the equivalence ratio, however, is similar for both the real
64 fuels and the neat components. All the fuels in Fig. 4.9 are seen to show the highest extinction stretch rate on the fuel rich side. This rich-shift is caused by the combined effects of positive stretch and sub-unity Lewis number for fuel rich mixtures. 4.4 Sensitivity Analysis A sensitivity analysis of the mass burning flux ( = ρ S ) with respect to the rate constants of the individual reactions is carried out for near stoichiometric fuel/air mixtures at the highest mixture preheat temperature of T u =470 K based on the kinetic model of Honnet et al. (2009). Figure 4.10(a) shows the normalized sensitive coefficients, ln(f o )/ ln(k i ), of the important reactions identified, where k i is the reaction rate constant of the i th reaction. The normalized sensitivity coefficient contains quantitative information on how each reaction rate constant affects the mass burning flux. As expected, the laminar flame speed is seen to be most sensitive to the reactions which involve either chain branching or termination reactions. Additionally, the CO oxidation reaction which is responsible for a significant amount of the heat release also shows a positive sensitivity. In order to conduct a similar sensitivity analysis for the extinction stretch rate, the eigenvalue in the plug-flow formulation is used, namely the radial pressure gradient H=( p/ r)/r. Note that the magnitude of H scales with the square of the stretch rate in the corresponding fully-developed potential flow (Kee et al., 1988). Hence, the normalized sensitivity of the radial pressure gradient with respect to the i th reaction is defined as S Hi =(k i /H)( H/ k i ). When evaluating S H,i at the extinction turning point, the positive (negative) value of S H,i indicates an increase (a decrease) in the extinction stretch rate with increasing k i. Figure 4.10(b) shows such results for a stoichiometric fuel/o 2 /N 2 counterflow flames with T u =400 K, based on the reaction mechanisms of Honnet et al. (2009). The controlling reactions identified for flame extinction are
65 generally consistent with the sensitivity analysis of the mass burning flux. An interesting exception is that the reactions involving phenoxy radicals are important for the extinction sensitivity. 4.5 Derived Cetane Number While the results of laminar flame speed and extinction stretch rates for Jet-A and alternative fuels are quite close to each other, the ignition results of the Derived Cetane Number (DCN) show more difference among these fuels. The DCNs are obtained using a Fuel Ignition Tester (FIT) manufactured by Waukesha Engine Dresser. Details about the FIT setup and measuring procedure can be found in (Hui et al., 2012a). The DCN values of jet fuels can provide a measure of their ignition characteristics in compression ignition engines, and are important parameters in engine design. The higher cetane number means shorter ignition delay time and more complete combustion of the fuel charge in the combustion chamber, which translates into a smoother running, better performing engine with more power and fewer harmful emissions. Table 4.2 lists the measured ignition delay times and the deduced DCNs of alternative jet fuels and Jet-A using an FIT, based on the ASTM D7170 method (An American National Standard, 2008). It can be seen that Jet-A s DCN is 49.35, much lower than most of alternative jet fuels whose DCNs are all above 60, except for the Sasol IPK which has as significant lower DCN of Cetane number mainly depends on the molecular composition of the fuel. From Fig. 4.1(a), it can be seen that Sasol IPK has very little n-paraffins in composition but large amounts of iso-paraffins and cyclo-paraffins, both of which are less reactive as compared to n-paraffins. Thus, IPK s low DCN is mainly due to a lack of the more reactive n-paraffin constituents
66 Table 4.2 also lists the IQT-DCN values and the engine CN values of some jet fuels taken from Bessee et al. (2011). It is seen that the IQT, FIT, and engine cetane ratings seem to be displaced from each other a few cetane numbers. This is because they use different techniques to determine the cetane rating. Nevertheless all the data are consistent in trend. Table 4.2 Measured ignition delays, Derived Cetane Numbers, and Cetane Numbers of various jet fuels Fuels Jet-A S-8 Sasol IPK HRJ-camelina Ignition Delay Time, ms (ASTM D7170) FIT-DCN (ASTM D7170) IQT-DCN (ASTM D6890) (Bessee et al. 2011) CN (ASTM D613) (Bessee et al. 2011) Since alternative jet fuels are currently used as blending components with conventional jet fuels, the blending DCN values are also of practical use and importance. By definition, CN is a linear volumetric blend of the blending contributions of all the different compositions present in the diesel fuel. Most of the models predict the blending CN based on linear combination of the cetane numbers of the components (Murphy et al., 2004), even though there is evidence that the linear assumption is not always correct (Ghosh and Jaffe, 2006). Figures 4.11(a)-(c) show the measured ignition delay times and the correlated DCN values of binary mixtures of alternative jet fuel and Jet-A using a Fuel Ignition Tester, as a function of the liquid volume percentage of Jet-A in the fuel blend. It is seen that there exists a linear relation between DCN (as well as ignition delay time) and Jet-A composition in the binary blend. As such, the nonlinear effect on the blending DCN is not prominent for
67 the blends of alternative jet fuels and Jet-A. This could be largely due to relatively simple compositions of alternative jet fuels, which are mostly n- and iso-paraffins. Figure 4.11(d) further plots the IQT-DCN results for S-8/JP-8 and Shell GTL/JP-8 blends reported in (Bessee et al., 2011) demonstrating the similar linear trend as the current FIT measurements. 4.6 Summary Laminar flame speeds and extinction stretch rates of Jet-A and three alternative jet fuels are determined in counterflow setup. The flame speed results show no discernible difference between alternative jet fuels and Jet-A, while extinction stretch rate data illustrated that alternative jet fuels are more resistant to extinction than Jet-A. Comparing to neat normal alkanes such as n-decane, and n-dodecane, the laminar flame speeds and extinction stretch rates of both Jet-A and alternative fuels are lower than those of normal alkanes. The lower reactivity of Jet-A and alternative fuels is due to their aromatic and branched alkane contents, respectively. The simulation results of laminar flame speed show good agreements with the experimental data for both Jet-A and S-8 with average deviations of 8% and 5%, respectively, while extinction stretch rates of Jet-A are significantly over-predicted by a maximum deviation of 50% by the mechanism of Honnet et al., (2009). Further sensitivity analysis shows a greater sensitivity to fuel composition for extinction stretch rate compared to laminar flame speed. Moreover, additional Derived Cetane Number data are provided for Jet-A and alternative jet fuels. The results suggested that ignition characteristics of jet fuels are more sensitive to fuel composition than flame propagation and extinction. Finally, the blending DCNs of binary mixtures of Jet-A and alternative jet fuels show a linear relation between DCN and blending fuel composition. Such a linear dependence of
68 DCN on blending ratio could be due to the similar volatility range and hydrocarbon distribution for alternative jet fuels
69 (a) (b) Figure 4.1 (a) Distribution of hydrocarbons in five SPK fuels (Moses, 2008) and (b) chromatograms of conventional and alternative jet fuels with n-paraffins identified
70 =0.8, Tu=400 K Reference Flame Speed (cm/s) Jet-A S-8 IPK Camelina Stretch Rate (1/s) (a) =1.0, Tu=400 K Reference Flame Speed (cm/s) Jet-A S-8 IPK Camelina Stretch Rate (1/s) (b) =1.2, Tu=400 K Reference Flame Speed (cm/s) Jet-A S-8 IPK Camelina Stretch Rate (1/s) (c) Figure 4.2 Reference flame speeds versus stretch rates for Jet-A/air, S-8/air, IPK/air, and camelina/air mixtures at 400 K preheat temperature and equivalence ratios of =0.8, 1.0, and
71 Laminar Flame Speed (cm/s) Laminar Flame Speed (cm/s) 80 Jet Fuel/Air Mixtures, T u =400K Jet-A S-8 IPK Camelina Equivalence Ratio, (a) Jet Fuel/Air Mixtures, T u =470K Jet-A S-8 IPK Camelina Equivalence Ratio, (b) Figure 4.3 Laminar flame speeds of Jet-A/air, S-8/air, IPK/air, and camelina/air mixtures as a function of equivalence ratio at preheat temperatures of (a) 400 K and (b) 470 K
72 Laminar Flame Speed (cm/s) Jet-A/Air Mixtures T u =470 K T u =400 K Jet-A Honnet et al Equivalence Ratio, Figure 4.4 Experimental measured and computed values of laminar flame speeds of Jet-A/air mixtures as a function of equivalence ratio at preheat temperatures of 400 K and 470 K
73 Laminar Flame Speed (cm/s) 100 S-8/Air Mixtures 90 T u =470 K T u =400 K S-8 Naik et al Equivalence Ratio, Figure 4.5 Experimental measured and computed values of laminar flame speeds of S- 8/air mixtures as a function of equivalence ratio at preheat temperatures of 400 K and 470 K
74 Laminar Flame Speed (cm/s) Comparative Laminar Flame Speeds T u =470 K T u =400 K Jet-A S-8 n-decane Equivalence Ratio, Figure 4.6 Laminar flame speeds of Jet-A/air, S-8/air, and n-decane/air mixtures as a function of equivalence ratio at preheat temperatures of 400 K and 470 K
75 Extinction Stretch Rate (1/s) Extinction Stretch Rate (1/s) Jet Fuel/O 2 /N 2 Mixtures, T u =400K [N 2 ]/([N 2 ]+[O 2 ]) = Jet-A S-8 IPK Camelina Equivalence Ratio, (a) Jet Fuel/O 2 /N 2 Mixtures, T u =470K [N 2 ]/([N 2 ]+[O 2 ]) = 0.86 Jet-A S-8 IPK Camelina Equivalence Ratio, (b) Figure 4.7 Extinction stretch rates of Jet-A/oxidizer, S-8/oxidizer, IPK/oxidizer, and camelina/oxidizer mixtures as a function of equivalence ratio at preheat temperatures of (a) 400 K and (b) 470 K
76 Extinction Stretch Rate (s -1 ) Maximum FlameTemperature (K) 1850 Aachen Surrogate for Jet-A, T u =400 K N 2 /(N 2 +O 2 )= Stretch Rate (s -1 ) (a) Jet Fuel/O 2 /N 2, T u =400 K Experimental, Jet-A Computational, Aachen Surrogate Equivalence Ratio, N 2 /(N 2 +O 2 ) = 0.84 (b) Figure 4.8 (a) Computed maximum flame temperature response to stretch rate variations and (b) comparison of experimental and computed extinction stretch rates for Jet-A flames at a preheat temperature of 400 K
77 Extinction Stretch Rate (s -1 ) Experimental Extinction Stretch Rates N 2 /(N 2 +O 2 ) = 0.84 T u =400 K Jet-A S-8 n-decane n-dodecane Equivalence Ratio, Figure 4.9 Comparative extinction stretch rates for Jet-A/oxidizer, S-8/oxidizer, n- decane/oxidizer, and n-dodecane/oxidizer mixtures at a preheat temperature of 400 K
78 Sensitivity for Mass Burning Flux ( 0.97, T u =470 K) HCO+OH<=>CO+H 2 O H+O 2 +M 1 <=>HO 2 +M C 2 H 3 +O 2 <=>CH 2 CHO+O 2CH 3 <=>C 2 H 5 +H OH+CH 3 <=>PXCH 2 2 +H 2 O C 2 H 2 +O<=>HCCO+H TXCH 3 2 +O 2 =>CO+OH+H HCO+M<=>CO+H+M CO+OH<=>CO 2 +H H+O 2 <=>OH+O Normalized Sensitivity Coefficient (a) Sensitivity for Extinction Stretch Rate ( 1.0, T u =400 K) H+O 2 +M<=>HO 2 +M C 6 H 5 O+H<=>C 6 H 5 OH HCO+OH<=>CO+H2O C 2 H 3 +O 2 <=>CH 2 CHO+O CH 3 CHO+H<=>CH 2 CHO+H 2 CH 3 +HO 2 <=>CH 3 O+OH HCO+M<=>CO+H+M C 6 H 5 O<=>C 5 H 5 +CO CO+OH<=>CO 2 +H H+O 2 <=>OH+O Normalized Sensitivity Coefficient 1 (b) Figure 4.10 Normalized sensitivity coefficients of Jet-A/air mixtures for (a) laminar burning flux and (b) extinction stretch rate. 1 M is third body. 2 PXCH 2 is methylene (single state). 3 TXCH 2 is methylene (triple state)
79 Ignition Delay Time (ms) FIT-DCN Value Ignition Delay Time (ms) FIT-DCN Value S-8/Jet-A Blends DCN Ignition Delay Percentage of Jet-A in Fuel Blend, Liquid Volume % (a) IPK/Jet-A Blends Ignition Delay DCN Percentage of Jet-A in Fuel Blend, Liquid Volume % (b)
80 IQT-DCN Value Ignition Delay Time (ms) FIT-DCN Value 5.5 Camelina/Jet-A Blends DCN Ignition Delay Percentage of Jet-A in Fuel Blend, Liquid Volume % (c) 70 S-8/JP-8 and Shell GTL/JP-8 Blends S-8/JP-8 Shell GTL/JP Percentage of JP-8 in Fuel Blend, Liquid Volume % (d) Figure 4.11 (a)-(c) Measured ignition delays and Derived Cetane Numbers of binary fuel blends in accordance with ASTM D7170, (d) measured Derived Cetane Numbers of binary fuel blends based on ASTM D6890 taken from the study of Bessee et al. (2011)
81 Chapter 5 Flame Propagation and Extinction of Jet-A and Surrogate Fuels 5.1 Background As gasoline and diesel fuels, jet fuel is a mixture of a large number of different hydrocarbons from different molecular classes including straight chain paraffins, branched chain paraffins, cycloparaffins, and aromatics (Maurice et al., 2001) (ExxonMobil, 2005). The complexity of jet fuel is shown in Fig. 5.1, which illustrates a typical hydrocarbon class distribution for Jet-A fuel. It should be noted that practical jet fuels derived from the refinery processing of crude petroleum are not the same. This is because that these fuels only need to meet broadly defined specifications, and their compositions vary not only with refinery and crude oil sources, but also with season and year of productions. Also, composition changes as fuel ages. Therefore, it is very difficult to control the consistency in fuel composition required for the purpose of research (Violi et al., 2002). As a consequence, the compositional complexity of real fuels creates a great challenge for combustion modeling, which could be utilized in engineering design for predicting fuel effects on new combustion technologies as well as for assessing the feasibility of the non-petroleum derived alternative fuels with legacy equipment. Surrogate fuels provide a pragmatic approach for modeling physical and chemical properties for real fuels. The concept of a surrogate mixture is to describe the important combustion kinetics of any particular real fuel in a model of manageable size that can still capture all the important combustion features of the real fuel. In particular, a surrogate fuel is defined as a mixture of a limited number of hydrocarbons whose composition can be formulated in order to best match real fuel properties, such
82 as physical properties, chemical properties, or even both (Edwards and Maurice, 2001). With their relatively small number of components, detailed chemical kinetics and transport properties of surrogate fuels can be developed to predict combustion performance of the target real fuels, such as ignition, flame stability, extinction, and emission characteristics, as long as the detailed kinetic models are validated against reliable fundamental combustion data. For real jet fuels in particular, such as JP-8 and Jet-A, extensive surrogate fuels have been proposed over the last decade. JP-8, a kerosene-type fuel, is widely used by US military to power aircraft and other high performance vehicles, while Jet-A is the commercial equivalent of military JP-8 and differs only by trace amounts of additives (Vann, 2008). In an early study, Schulz (1991) first proposed a 12-component surrogate fuel for JP-8, and compared the oxidative stability of the surrogate with real fuel. Gueret et al. (1991) compared the oxidation process of a kerosene fuel with a three-component surrogate fuel, composed of 79% n-undecane, 10% n- propylcyclohexane, and 11% 1,2,4-trimethylbenzene by mole, in a jet stirred reactor, and developed a quasi-global chemical kinetic mechanism. Using n-decane as single component surrogate, Dagaut et al. (1994) is able to reproduce experimental data of Jet-A1 in a jet stirred reactor, and their results show a strong analogy between n- decane and kerosene oxidation kinetics. Humer et al. (2007) defined three surrogate fuels (made up of n-decane, n-dodecane, methylcyclohexane, toluene, and o-xylene) and compared their extinction and autoignition with JP-8, Jet-A, and other two surrogates proposed by Violi et al. (2002) and Agosta (2002), respectively. Recently, Honnet et al. (2009) proposed the Aachen surrogate (20% 1,2,4-trimethylbezene and 80% n-decane by weight) for Jet-A and the associated semi-detailed mechanism. Later, Dooley et al. (2010) first proposed a three-component Jet-A surrogate, composed of n
83 decane, iso-octane, and toluene, and compared the surrogate with Jet-A for a variety of experimental data, showing a good agreement in terms of experimental comparison between the surrogate and the target real fuel. Since further analysis suggested that large molecular weight hydrocarbons are required to match the typical threshold sooting index and molecular weight of Jet-A, Dooley et al. (2012) subsequently formulated a four-component surrogate, composed of n-dodecane, iso-octane, 1,3,5- trimethylbenzene, and n-propylbenzene. The appropriateness of this four-component surrogate formulation is demonstrated through the experimental measurement of various gas phase combustion kinetic phenomena of the surrogate mixture and of the target Jet-A fuel (Dooley et al., 2012). The latest two surrogates proposed by Dooley et al. (2010, 2012) are developed under a multi-university research initiative (MURI) program, which is aimed to develop a methodology for the formulation of surrogate fuels to emulate gas phase combustion kinetic phenomena of real jet fuels. Prior to these two surrogates, though a considerable amount of work has been devoted to surrogate formulation, most of these studies focused on the surrogates that can only reproduce one or some, tested or assumed parameters of a real fuel of interest; and there still remains no well proven procedure for surrogate fuel formulation and no comprehensive testing of the combustion behaviors of surrogate fuel to that of intended target fuel over a wide range of combustion phenomena or reaction conditions. Therefore, the overall goal of the MURI effort has been developing concepts, metrics, advanced metrology and cross-validated critical experimental data along with new fundamental insights to produce surrogate mixtures that would accurately reflect the physical and chemical kinetic properties of each specific real fuel sample under multi-phase combustion conditions relevant to aircraft gas turbine applications (Dryer et al., 2012). The two
84 mixtures of surrogate components are investigated to prove the proposed surrogate formulation concept. The surrogate mixture proposed by Dooley et al. (2010), composed of n-decane/iso-octane/toluene=42.7/33.0/24.3 mole %, is denoted as the 1 st generation MURI surrogate, while the surrogate mixture proposed by Dooley et al. (2012), composed of n-dodecane/iso-octane/1,3,5-trimethylbenzene/n-propylbenzene =40.41/29.48/7.28/22.83 mole %, is denoted as the 2 nd generation MURI surrogate. This chapter is part of the MURI program that studied the premixed flame properties of laminar flame speed and extinction limit of these two surrogate mixtures and their target jet fuel of Jet-A POSF In the following sections, we will first introduce the strategy with which the two surrogates are formulated, and then present the flame results by comparing the experimentally determined laminar flame speeds and extinction stretch rates of the two MURI surrogates and Jet-A. 5.2 Surrogate Formulation In general, in order to best emulate the combustion properties of a real fuel, the selection of its surrogate components typically proceeds by selecting one or more species to represent each of the chemical classes found in the real fuel. However, due to the complexity of the real fuels, this method can result in a quite large number of surrogate components if one tries to cover all chemical structures of target real fuel, thus making the kinetic modeling much more difficult as the cost of computation increases significantly with number of surrogate components. In addition, the information of the real fuel composition has to be required before the surrogate components can be selected. Recognizing that the combustion kinetic phenomena is principally influenced by the capability of molecular structure to produce important radical species that affect the main heat releasing and radical chain branching reactions, such as CO+OH CO 2 +H and H+O 2 O+OH respectively, in the high
85 temperature kinetics of large hydrocarbons (Westbrook, 2000), the reproducing of these important radicals can be considered as fundamental emulation target in order for surrogate fuels to emulate real fuel combustion behaviors. Based on this principle, the concept of distinct chemical functionality can be introduced into the scheme of surrogate formulation Concept of Distinct Chemical Functionality The distinct chemical functionalities proposed by Dooley et al. (2012) are defined by thermochemical kinetic molecular environments that are significantly different in character in terms of radical production/consumption. For example, there are some real fuel components that contain an n-alkyl molecular functionality. This n-alkyl can be combined with an aromatic, a cycloalkyl, alkenyl, or isomerized alkyl to form a molecule. After the initial break of the molecule, a generic collection of n-alkyl radicals can be formed, and the subsequent fate to these n-alkyl radicals is dependent on thermochemical kinetic molecular environment local to the radical site. Similar local thermochemical kinetic molecular environment will undergo similar molecular processes to form a similar distribution of product. The concept of the distinct chemical functionality in high temperature oxidation is shown in Fig. 5.2 (taken from Dooley et al., 2012). It can be seen that though there are many generic chemical kinetic functionalities in the real fuel level, after initial reaction only three types of chemical functionalities are formed including n-alkyls, iso-alkenlys, and benzyls. Table 5.1 lists the surrogate component candidates that can produce these three chemical functionalities upon their individual oxidation. Based on the concept of the distinct chemical functionality, surrogate components only need to reproduce the chemical kinetic functionalities of the target fuel. Therefore, not all of the candidates are necessary to be included in the surrogate mixture, since many of them produce the
86 same chemical functionality. For example, the cycloalkanes are classified as nondistinct chemical functionalities from normal alkanes, and can be excluded from the surrogate component selection once a normal alkane is selected, even though there is a large amount of cycloalkanes found in the real fuels. Another advantage of using this concept is that no detailed composition information of the real fuel is required for the surrogate component selection, since the selection only focuses on matching the important intermediate species and radicals of the surrogate with those of the target fuel. However, there is an implicit assumption under this concept that there are no important reactions between high molecular weight radical species and fuel components. Table 5.1 Surrogate component candidates selected for the MURI research. Component Formula Structure n-heptane C 7 H 16 n-decane C 10 H 22 n-dodecane C 12 H 26 iso-octane C 8 H 18 Methylcyclohexane C 7 H 14 Toluene C 7 H 8 n-propylbenzene C 9 H 12 1,3,5-Trimethylbenzene C 9 H
87 1-Methylnaphthalene C 11 H Combustion Property Targets The strategy for specifying the mixture of surrogate components that would best emulate the global combustion properties of the real fuel relies on matching the combustion properties targets including hydrogen/carbon ration (H/C), derived cetane number (DCN), threshold sooting index (TSI), and average molecular weight (MW ave ). Each of these targets is designed to establish a correlation of chemical structure of the surrogate mixture that would reproduce important gas phase combustion kinetic related phenomena that numerical models must reproduce, such as laminar flame speed, extinction, ignition delay, etc. a. Hydrogen/Carbon Molar Ratio, H/C The H/C ratio defines the ratio of water and carbon dioxide formed from the combustion of fuel. With the molecular weight they together determine the enthalpy of reaction and the adiabatic flame temperature. Thus, it strongly affects the flame phenomena that are dominated by the flame temperature like the flame propagation. The H/C ratio varies significantly from alkanes ( ) to aromatics (1-1.4), while the jet fuels have a H/C ratio range of b. Derived Cetane Number, DCN The DCN provides a measure of fuel ignition characteristic in compression ignition engine. The DCN value is determined by correlating ignition delay measured in particular experimental procedure, and is a convenient way for reporting absolute ignition delay data for fuels. The use of DCN for the surrogate formation is a means of
88 representing the relative global reactivity amongst the fuels, components, and mixtures tested. c. Threshold Sooting Index, TSI The threshold sooting index is defined by Calcote and Manos (1983) as: Molecular weight TSI = a ( ) + b Smoke point (5.1) where the smoke point is the maximum smoke free laminar diffusion flame height (mm) (Olson et al., 1985), molecular weight is in g/mol, and a (mol mm/g) and b (dimensionless) are experimental constants. The TSI is a macro measure of the tendency of a fuel to form soot under diffusive/mixing limited conditions. It is of practical importance that a surrogate mixture emulates the sooting tendency of a target fuel. In addition, it also limits the aromatic content in the surrogate fuel that should be similar to that in the real fuel as Yang et al. (2007) have demonstrated that TSI is strongly dependent on aromatic contents. d. Molecular Weight, MW ave The diffusion properties of gas phase fuel are strongly correlated with the average molecular weight. Thus, in order to emulate the diffusive properties of real aviation fuels in gas phase flame environments, a surrogate fuel must be of similar average molecular weight. The H/C ratio along with the molecular weight is also strongly correlated with the average heat of combustion per mole, since for large hydrocarbons the number of moles of fuel species is small relative to the number of moles of oxidation product species. And it is also a parameter implicit in the determination of the TSI of molecular mixtures, and a determining factor in terms of volumetric air flow required for a specific combustion stoichiometry
89 Table 5.2 lists these four combustion targets of Jet-A POSF 4658, 1 st and 2 nd generation MURI surrogates. It can be seen that the 2 nd generation MURI surrogate matches the targets of Jet-A much better than the 1 st generation MURI surrogate. Table 5.2 Combustion property targets of Jet-A, 1 st and 2 nd generation MURI surrogates. DCN H/C MW g/mol TSI Target Jet-A POSF ± st generation surrogate nd generation surrogate Surrogate Component Selection To obtain the optimum surrogate mixture composition, a mapped surface of DCN as a function of surrogate composition is generated first. The H/C ratio, TSI, and MW ave of a given mixture with certain DCN value can be calculated through some analytical expressions. Then the surrogate composition that can best reproduce all of the real fuel combustion targets can be obtained by generating a probability function that depends on each property target functional relationship. The first derivatives of the probability function with respect to each surrogate component are derived and a normalized function composed of the sum of the squares of the first derivatives is minimized to obtain the global optimal composition. Details about this methodology can be found in the MURI final report (Dry et al., 2012). Based on this methodology, two surrogate mixtures are proposed, the first and second generation surrogate mixtures. The 1 st generation surrogate composed of n- decane, iso-octane, and toluene is shown to emulate very well the Jet-A (POSF 4658) fuel in terms of predicting homogeneous auto-ignition characteristics, species evolution, and diffusion flame properties; however, it could not encompass the average
90 molecular weight and sooting property targets of Jet-A (POSF 4658), suggesting large molecular weight components are required. The 2 nd generation mixture composed of n-dodecane, iso-octane, 1,3,5-trimethylbenzene, and n-propylbenzene is found to not only match all four the above property targets of Jet-A (POSF 4658), but also exhibit the same global combustion behaviors of the fully prevaporized Jet-A across a wide range of fundamental experiments. In the next result section, experimentally measured laminar flame speeds and extinction stretch rates of 1 st and 2 nd generation surrogate with comparison to those of Jet-A (POSF 4658) are presented. And people of interest are referred to the studies of Dooley et al. (2010, 2012) and Dry et al. (2012) for other experimental results, such as ignition delay, shock tube speciation, flow reactor oxidation, and diffusion flame studies. 5.3 Results First, it should be noted that laminar flame speeds presented in this chapter are all determined by using the linear extrapolation method. Figure 5.3 compares the atmospheric laminar flame speeds of Jet-A, 1 st and 2 nd generation surrogates at preheat temperatures of (a) 400 K, and (b) 470 K. It can be seen that at both 400 and 470 K preheat temperatures the two surrogates share very closely the laminar flame speeds with the target Jet-A fuel. The laminar flame speed behavior with equivalence ratio for both surrogate and real fuels is typical to that observed for other hydrocarbons that the peak of the flame speed is at equivalence ratio of 1.1. It can also been that with preheat temperature increasing from 400 K to 470 K, the laminar flame speeds increase about 30%, consistent with previous studies on both pure components (Kumar and Sung, 2007, 2010b) and real fuels (Kumar et al., 2010). The laminar flame speed is strongly dictated by the flame temperature. In the methodology of surrogate formation, flame
91 temperature is intended to be described through the sharing of H/C ratio. Thus the close emulation of this data set gives some indication as to how precisely this combustion property target must be matched in a surrogate fuel that properly emulates the target real fuel. As shown in Table 5.2, Jet-A POSF 4658 is of H/C 1.96, the 2 nd generation of surrogate is of H/C 1.95, and the 1 st generation surrogate is of H/C 2.01, yet the laminar flame speeds are essentially indistinguishable. Figure 5.4 compares the extinction stretch rates of Jet-A, 1 st and 2 nd generation surrogates at preheat temperatures of (a) 400 K, and (b) 470 K. Noting that the diluted air is made of 86% N 2 and 14% O 2. It is expected that the extinction stretch rates increase with preheat temperature. At both 400 and 470 K preheat temperatures, both surrogates and Jet-A share very similar extinction stretch rates. Though within the experimental uncertainties, the extinction stretch rates show a greater sensitivity than the laminar flame speeds that the 1 st generation surrogate seems to have the highest extinction stretch rate, followed by the 2 nd generation surrogate, and Jet-A has the lowest extinction stretch rate. The differences in extinction limit for the surrogates and Jet-A fuel are relatively minor, in the worst =1.3 case, the extinction limit is measured to be 494 s -1, 508 s -1 and 541 s -1 at 470 K (306 s -1, 338 s -1 and 358 s -1 at 400 K) for Jet-A, 2 nd and 1 st generation surrogate flames, respectively. This corresponds to extinction limits similar to within 10% in the worst case scenario. Though it is accepted that the rate of mass diffusion is a relatively insensitive parameter in inducing extinction in premixed flames (Holley et al., 2007), it is apparent that the ordering of these slight disparities are in order with the modest differences in molecular weight between the three fuels. Nonetheless, it may be concluded that both surrogate fuels emulate the effect of equivalence ratio and the effect of unburned gas temperature observed for the target real fuel very well. Moreover, the absolute values of the
92 premixed extinction limit of Jet-A determined in the twin-flame configurations are reasonably emulated by both intended surrogate fuels. The premixed flame results of laminar flame speed and extinction stretch rate show quite similarity between the two MURI surrogates and Jet-A (POSF 4658), which indicates that the small differences in average molecular weight between the 1 st generation surrogate and the target Jet-A (~20 g/mol) are unimportant under the premixed conditions. Such may have been expected given the analysis presented by Holley et al. (2007) but this is not true under conditions where mass diffusive effects are more controlling as shown previously (Dooley et al., 2010). Secondly, the premixed flame data provide a further indication that the high temperature chemical kinetics that dictate flame phenomena may be properly prescribed by the proposed surrogate formulation methodology. 5.4 Summary A methodology that is developed under the MURI program to formulate reliable surrogate fuels for practical jet fuels is introduced. It is found that matching the real fuel combustion property targets hydrogen/carbon molar ratio (H/C), derived cetane number (DCN), threshold sooting index (TSI), and average mean molecular weight (MV ave ) through proper choice of surrogate components and their mixtures resulted in nearly identical global combustion behaviour of the real fuel. Further premixed flame data including laminar flame speeds and extinction stretch rates are presented to validate two surrogates that are developed under this methodology. The results show that both surrogates can emulate very well the combustion properties of the target fuel in the premixed flame environments, though there are some minor differences
93 observed in the extinction limit results, which may be attributed to the differences in the average molecular weight
94 ND 1% misc 2% cyclo-paraffin 20% n-paraffin 28% naphthalenes 2% alkylbenzenes 18% i-paraffin 29% Figure 5.1 World-wide average molecular class distribution of Jet-A (Shafer et al., 2006)
95 Figure 5.2 Schematic diagram of real fuel oxidation and concept of distinct chemical functionality (Dooley et al., 2012)
96 Laminar Flame Speed (cm/s) Laminar Flame Speed (cm/s) Comparison of Laminar Flame Speeds, T u =400K and P=1 atm Jet-A 1st 2nd Equivalence Ratio, (a) Comparison of Laminar Flame Speeds, T u =470K and P=1 atm Jet-A 1st 2nd Equivalence Ratio, (b) Figure 5.3 Comparison of laminar flame speeds of Jet-A, 1 st and 2 nd generation MURI surrogates at preheat temperatures of (a) 400 K, and (b) 470 K
97 Extinction Stretch Rates (s -1 ) Extinction Stretch Rates (s -1 ) 500 Comparison of Extinction Limits, T u =400 and P=1 atm N 2 /(N 2 +O 2 )= Jet-A 1st 2nd Equivalence Ratio, (a) 700 Comparison of Extinction Limits, T u =470 and P= 1atm 600 N 2 /(N 2 +O 2 )= Jet-A 1st 2nd Equivalence Ratio, (b) Figure 5.4 Comparison of extinction stretch rates of Jet-A, 1 st and 2 nd generation MURI surrogates at preheat temperatures of (a) 400 K, and (b) 470 K
98 Chapter 6 Flame Propagation and Extinction of Selected Aromatic Hydrocarbons 6.1 Background Gaining a basic understanding of combustion characteristics of aromatic hydrocarbon fuel components is important to understand real transportation fuel combustion characteristics, since these fuels contain substantial amounts of aromatics (Speight, 2007). Aromatic compounds increase the energy density of transportation fuels and are of significance especially in automotive fuels to improve antiknock performance as a result of their high octane ratings (Walsh, 1949). It has also been demonstrated that the presence of aromatic components in the fuel blends can have a significant impact on the global combustion responses, such as laminar flame speed and extinction limit [e.g., (Hirasawa et al., 2002) (Won et al., 2010) (Kumar et al., 2010)]. In other venues, the formations of polyaromatic hydrocarbons (PAHs) from aromatic combustion are important as precursors to soot formation (Frenklach et al., 1985). Though aromatics can be entirely removed from future air transportation fuels, the hardware legacy in this field appears to require a minimum content of aromatic species to maintain elastomer sealing qualities (Edwards et al., 2010). Therefore, it is of fundamental and practical significance to refine the current understanding of aromatic combustion characteristics and their coupling performances with other fuel structures present in the transportation fuels. Extensive studies [e.g. (Dagaut et al., 2006), (Lindstedt and Maurice, 2000), (Violi et al., 2002)] have focused on constructing reliable surrogates for transportation fuels, including gasoline, diesel, and kerosene jet fuels. The objective is to determine a specific mixture composition to best emulate desired characteristics of a specific real
99 fuel of interest. The simplest aromatic, benzene, is limited as a component in fuels as a result of its carcinogenic characteristics. On the other hand, toluene is a major component found in gasoline, and the reactions describing its pyrolysis and oxidation provide much of the submodel components for describing more complex aromatic combustion chemistry (Metcalfe et al., 2011). As we have discussed in Chapter 5, the 1 st generation MURI surrogate composed of n-decane/iso-octane/toluene is shown to emulate very well the target Jet-A fuel in terms of predicting homogeneous auto-ignition characteristics, species evolution, and diffusion flame properties. Matching of surrogate fuel mixtures with real fuel combustion property targets of hydrocarbon/carbon (H/C) ratio, derived cetane number (DCN), threshold soot index (TSI), and average molecular weight are utilized to emulate the real fuel behaviors. However, the study of 1 st generation MURI surrogate showed that larger molecular weight components are required to match the typical TSI and average molecular weight of jet fuels. Thus in the follow-on research the 2 nd generation MURI surrogate mixture containing larger molecular weight alkyl aromatics, specifically n-propylbenzene and 1,3,5-trimethylbenzene is proposed. Other trimethyl benzenes have also been used by other investigators in constructing surrogate mixtures (Honnet et al., 2009). For all of the above practical drivers, it is essential to improve understanding of the combustion chemical kinetics of neat toluene and the C 9 aromatics. Without doubt, the oxidation of these aromatics is much less well characterized than the oxidation of normal and branched alkane components found in real fuels. A brief overview of combustion studies on the C 9 alkyl aromatics appearing in the literature is provided as follows. Brezinsky (1986) studied the oxidation of n- propylbenzene in the plug-flow reactor. Dagaut et al. (2002) studied the oxidation of
100 n-propylbenzene in the jet-stirred reactor under atmospheric pressure and over the temperature range from 900 to 1250 K and developed a detailed chemical kinetic model. Farrell et al. (2004) reported the laminar burning velocities of various aromatics, including n-propylbenzene, 1,2,4-trimethylbenzene, 1,3,5- trimethylbenzene, etc., at the preheat temperature of 450 K and pressure of 3 atm. However, the burning velocity data of (Farrell et al., 2004) are not stretch-corrected. Honnet et al. (2009) developed a kinetic model for a kerosene surrogate composed of n-decane and 1,2,4-trimethylbenzene. Recently, a semi-detailed n-propylbenzene kinetic model has been proposed by Won et al. (2011) to investigate diffusive extinction. Despite these previous efforts, a deficiency of the experimental data essential to developing comprehensive reaction models that are predictive over a broad range of experimental venues and combustion continues to exist. This is especially true for stretch-corrected laminar flame speed data for these aromatic fuel constituents. To this end, the objective of this chapter is to provide fundamental combustion properties, namely laminar flame speeds and extinction stretch rates of three C 9 aromatics, n-propylbenzene (n-pb), 1,2,4-trimethylbenzene (1,2,4-TMB), and 1,3,5- trimethylbenzene (1,3,5-TMB). The three C 9 H 12 isomers are expected to have different combustion characteristics primarily as a result of their different alkyl substitutions, as their adiabatic flame temperatures and mass diffusive properties are similar. In addition to the above three aromatic hydrocarbons, this work also includes additional study of toluene, the simplest alkyl aromatic. The molecular structures of the aromatic components studied herein are shown in Table 6.1. The comparison of these isomers along with toluene allows for investigating molecular structure effects on flame propagation and extinction of premixed stretched flames. Further, by comparing the existing kinetic model predictions against the present experimental data, we aim to
101 provide more insight into the controlling reactions within these models that most strongly affect flame propagation and extinction. Table 6.1 Molecular structures of the aromatic components investigated 6.2 Kinetic Models The kinetic models used to simulate toluene flame speeds are taken from the work of Metcalfe et al. (2011). This detailed chemical kinetic model is composed of 328 species and 1888 elementary reactions. In the same paper, a reduced model is derived from the detailed one, consisting of 139 species and 525 reactions. The reduced model of Metcalfe et al. (2011) is reported to be valid over the temperature range of K. As shown in due course, this reduced model is found to well reproduce computed laminar flame speeds of toluene/air mixtures using the detailed model. Thus, for ease of computation, the reduced model is employed in generating the flame response curves for determining the extinction limits. The toluene model also served as the base model for the n-pb model, recently published by Won et al. (2011). The n-pb submodel (that is added to the toluene model of Metcalfe et al. (2011) is composed of 8 species and 50 reactions that describe the pathways of fuel decomposition, hydrogen abstraction, and alkyl radical and other intermediate consumption. As described by Won et al. (2011), the n-pb submodel is constructed by an assumed direct analogy to toluene oxidation kinetics for the reactions occurring from the benzyl-type position and by analogy to those of propane
102 for the reactions occurring at the alkyl positions. The so derived n-pb model is tested against diffusion flame extinction results in Won et al. (2011) and some shock tube ignition delay data in Wang et al. (2011). However, no comparisons have been made previously against premixed flame data. For 1,2,4-TMB flames, the kinetic model is taken as that developed for representing the Aachen kerosene surrogate (Honnet et al., 2009). This model, which includes semi-detailed descriptions of n-decane and 1,2,4-TMB chemistry, is composed of 118 species and 527 reactions. 6.3 Results Figure 6.1 compares the atmospheric laminar flame speeds of n-pb/air, toluene/air, 1,2,4-TMB/air, and 1,3,5-TMB/air mixtures at T =400 K and 470 K, and P=1 atm. It can be seen that all the laminar flame speeds increase by about 30% with T increased from 400 K to 470 K. Among the aromatics, n-pb has the highest laminar flame speed, followed by toluene and the two TMBs. Farrell et al. (2004) reported the same flame speed ranking for these four aromatic components under experimental conditions at T =450 K and P=3 atm. Their results also showed that the laminar burning velocities (without stretch correction) of 1,2,4-TMB are slightly higher than those of 1,3,5-TMB. However, within the experimental uncertainty there is no discernible difference observed in laminar flame speed results of these two TMBs in the present study. Since the laminar flame speeds are mostly dominated by the flame temperature. Figure 6.2 shows the computed adiabatic flame temperatures of n-pb/air, toluene/air, 1,2,4-TMB/air mixtures. It can be seen that temperature ranking is in the order of toluene> n-pb>1,2,4-tmb, but the difference in adiabatic flame temperature is quite small, especially at fuel lean conditions. Despite that the adiabatic flame temperatures of toluene/air mixtures are somewhat higher than those of n-pb/air mixtures, the
103 laminar flame speeds of toluene/air mixtures are lower than those of n-pb/air mixtures. Also, the diffusive properties of the four aromatic hydrocarbons are quite similar. Therefore, it can be concluded that the differences in laminar flame speeds of the aromatic components are largely caused by the different oxidation kinetics of each fuel as a result of the different alkyl substitution structures. Comparing the two mono alkyl substituted benzenes, n-pb and toluene, a significant difference between their oxidation mechanisms is the pathway of fuel molecule disintegration. As also suggested by Dagaut et al. (2002), the high reactivity of n-pb is due to the species produced from reactions of the longer side-chain of n-pb. As for toluene and TMBs, their structure difference lies in the degree of the methyl substitution. It is reasonable to expect radical regeneration processes in TMB flames to be slower than in toluene flames as the TMBs have more numerous opportunities to produce the uniquely stable benzyl type radicals that dominate the reactivity of both systems (da Silva and Bozzelli, 2010). Thus, in addition to the effect of adiabatic flame temperature, the chemical kinetic effect due to their structure difference also contributes to the difference in laminar flame speeds of toluene and TMBs. Other studies (Johnston and Farrel, 2005) on xylenes also showed that the m-xylene (2,4- dimethylbenzene) has a lower flame speed than toluene. These results suggest that the degree of the substitution can have a strong effect on fuel reactivity. Moreover, the position of substitution can also play an important role in affecting the fuel reactivity. It has been reported in previous flame studies of Farrell et al. (2004), Johnston and Farrel (2005), and Won et al. (2011) that o-xylene is more reactive than p-xylene and m-xylene. However, to quantify and isolate the influence of the degree and position of substitution, more experimental data are still required for a systematic comparison of the aromatic components
104 In the flame extinction experiments, two different modes of flame extinction have been observed. In the first mode, the lean flames are extinguished with a finite separation distance between each other, as shown in Fig. 6.3(a). In the second mode, the rich flames merged into each other before extinction occurred as shown in Fig. 6.3(b). These different modes of flame extinction result from the coupled effects of positive stretch rate and non-unity Lewis number ( Le ) (Law, 1989) (Tsuji and Yamaoka, 1982). For the aromatic hydrocarbons studied herein, the effective Le of lean (rich) mixtures are greater (smaller) than unity. When the positive stretch rate is imposed on the lean flame (Le>1), flame temperature decreases because thermal energy loss from the reaction zone exceeds chemical energy gain due to mass diffusion of the deficient reactant (Law, 1989). Flame extinction occurs when the flame temperature drops to the extent that the flame cannot sustain itself. Vice-versa when the mixture is fuel rich (Le<1), the combined effect of positive stretch and disparate diffusion of mass and energy leads to an increase in flame temperature and enhanced combustion until the twin flames move towards the stagnation plane and merge into each other. Further increasing the stretch rate reduces flow residence time thus decreasing the flame temperature. The flame extinction eventually occurs due to the incomplete combustion. Figure 6.4 depicts the experimentally determined extinction stretch rates of n- PB/oxidizer, toluene/oxidizer, 1,2,4-TMB/oxidizer, and 1,3,5-TMB/oxidizer mixtures as a function of equivalence ratio at T =400 K and P=1 atm. It can be seen that all the extinction stretch rates peak on the fuel rich side at ~1.4 which is much richer than the value where the respective laminar flame speeds peak. Among all the aromatics, n- PB has the highest resistance to extinction, followed by toluene and TMBs in descending order. Comparing the extinction limits of n-pb and toluene, n-pb is
105 consistently higher than toluene and the difference becomes larger when the fuel/oxidizer mixtures become rich. Based on the laminar flame speed results, it is seen that n-pb flame is more reactive, resulting in shorter characteristic reaction time than toluene flame, thus it can sustain higher stretch rate. The extinction limits of the TMBs are substantially lower than those of n-pb and toluene, which is consistent with the laminar flame speed results that TMBs are less reactive than n-pb and toluene. Between the two TMBs, even though the laminar flame speed results show no discernible difference, the extinction limit results, while within the experimental uncertainty, nevertheless show that extinction stretch rates of 1,2,4-TMB are slightly higher than those of 1,3,5-TMB. Same trend has been reported in the diffusion flame extinction experiments of Won et al. (2011). 1,3,5-TMB has a more symmetrical structure compared to 1,2,4-TMB, which could be the reason that 1,3,5-TMB is more stable. In addition, it is conjectured that for 1,2,4-TMB some interaction between the radicals formed at the 1 and 2 positions can -scission to produce a quantity of radical pools, while the isolation of the methyl configurations in 1,3,5-TMB would not allow for such an interaction and hence would rely more on bi-molecular reactions to propagate the radical chains. Though the detailed chemistry for the two TMBs is still unclear, the experimental results suggest that the position of the substitution can have an impact on the flame extinction response of the fuel isomers. Figures compare the measured laminar flame speeds with computed ones of toluene/air, n-pb/air, and 1,2,4-TMB/air mixtures at T =400 and 470 K and P=1 atm. It can be seen that the computed values by each model agree fairly good with experimental data with average deviations of 4%, 8%, and 7% for the flame speeds of toluene, n-pb, and 1,2,4-TMB, respectively. However, all the models under-predict the laminar flame speeds on the lean side. It is further noted that the use of the detailed
106 and reduced toluene models of Metcalfe et al. (2011) yields closely-matched laminar flame speeds for both toluene and n-pb simulations. This close agreement also justifies the use of the reduced toluene model in extinction calculations. Figures compare the measured extinction stretch rates with computations of toluene/oxidizer, n-pb/oxidizer, and 1,2,4-TMB/oxidizer at T =400 K and P=1 atm, respectively. Note again the oxidizer in this extinction stretch rate experiments is composed of 84% N 2 and 16% O 2 (by mole). It can be seen that the n-pb model, the combination of the reduced toluene model of Metcalfe et al. (2011) and the n-pb submodel of Won et al. (2011) over-predicts the extinction stretch rates with a maximum deviation less than 20%. A similar over-prediction is also reported in the diffusion flame extinction study by Won et al. (2011). Though the reduced toluene model predicts the peak at =1.3, leaner than the experimental value of =1.4, the overall prediction is in reasonable agreement with the experimental data. The 1,2,4- TMB model of Honnet et al. (2009) substantially under-predicts the extinction stretch rates with a maximum deviation of 40%. Similar trends are found in diffusion flame extinction study by Won et al. (2011). Their results suggested that the under-prediction of 1,2,4-TMB model is mainly due to the deficiency of its toluene submodel at atmospheric pressure. Despite the deficiency of these models, a reasonable prediction of the present experimental data has been shown in Figs Further evaluations of these models against mechanistically revealing experimental data would be enlightening in confirming the veracity of each model. Such further analyses can be used to provide more definitive kinetic analyses of these flames. 6.4 Sensitivity and Flux Analysis Figures show the normalized sensitivity coefficients of mass burning flux and extinction limit for toluene, n-pb, and 1,2,4-TMB flames at stoichiometric
107 condition, T =400 K, and P=1 atm. Not surprisingly, the main chain-branching reaction H+O 2 O+OH is the most sensitive reaction in both laminar flame speed and extinction limit simulations for all the fuels. The reaction of CO+OH CO 2 +H, which is responsible for most of the heat release, also shows a great positive sensitivity. It can also be seen that extinction sensitivities are much larger than laminar flame speed sensitivities, emphasizing the importance of kinetics in simulating the extinction limit. To provide more insight, a reaction path analysis is performed on each of the present models to identify the key species that are responsible for the predicted reactivity of each aromatic. The main difference in the present kinetic models is from the consumption pathways available to the fuel structure due to the different alkyl substitutions. Recognizing this and that each model has a similarity after the fuel molecule breaks down to smaller aromatic fragments, the path analysis is only performed for the initial few steps of the oxidation process. Figure 6.14 plots the propyl substitution breaking pathway of n-pb premixed flames at =1.0 and T =400 K. Both freely-propagating and near-extinction flames are analyzed and compared. The initial fuel breakdown is dominated by the hydrogen abstraction reactions, the top three channels in Fig. 6.14, which account for 73% of total fuel consumption in freely propagating flame and 87% of total fuel consumption in near-extinction flame. The rest fuel consumption is through the unimolecular decomposition reactions, with the major channel being plotted in Fig This is expected in premixed flames as hydrogen abstraction reactions are more important than unimolecular decomposition reactions in the initial fuel breakdown. The abstraction can occur from primary, secondary, and the benzylic-type C-H bonds, yielding three phenylpropyl (C 6 H 5 C 3 H 6 ) isomers. It is noted that the n-pb submodel of Won et al. (2011) is constructed by an assumed direct analogy to the equivalent
108 processes of propane. As such, by β-scission these phenylpropyl radicals are described to decompose unimolecularly to form either benzyl (C 6 H 5 CH 2 ) and ethylene (C 2 H 4 ), phenyl (C 6 H 5 ) and propene (C 3 H 6 ), or styrene (C 6 H 5 C 2 H 3 ) and methyl (CH 3 ) radical. The styrene submodel is then described to ultimately result in phenyl and acetylene (C 2 H 2 ). Recognizing the importance of styrene as an intermediate and the simplicity of the present model in describing styrene oxidation chemistry, further attention to the mechanism of styrene oxidation is warranted. It is expected that the production of these C 2 species and the radicals that accompany their formations are responsible for n-pb s relatively higher reactivity shown in the laminar flame speed and extinction limit in premixed combustion. Although not shown in the figure, the same flux analysis of toluene and 1,2,4-TMB flames also shows that most of the fuel breaking pathways are through the hydrogen abstraction reactions. However, due to their methyl substituted structures, there are no opportunities for beta-bond scission reactions and thus no C 2 species that can be formed in the early fuel consumption. Moreover, after the initial radical attack on toluene or TMB, oxidation process leads to the formation of benzylic radicals. These benzylic-type radicals are resonantly stabilized and do not have the opportunity to propagate the radical chain by beta-bond scission reactions. To further quantify the presence of the C 2 and benzylic radicals in flames, Fig compares the computed profile of the combined mass fractions of C 2 H 2 and C 2 H 4 radicals in the freely propagating flames at =1.0 and T =400 K. It can be seen that the total mass fraction of the key C 2 species in the n-pb flame is much higher than those in toluene and 1,2,4-TMB flames. The excess of C 2 species in the n-pb flame is found to be produced during the initial propyl substitution breakdown. Figure 6.16 shows spatially-resolved benzylic radicals, C 6 H 5 CH 2 in the n-pb and toluene flames as well
109 as C 6 H 5 C 3 H 6 in the 1,2,4-TMB flame, under the same conditions. It is seen that the benzylic radical in the 1,2,4-TMB flame is notably higher than those in the n-pb and toluene flames. The high concentration of benzylic radicals in the 1,2,4-TMB flame could explain its lower reactivity observed in the experiments. 6.5 Summary Laminar flame speeds and extinction stretch rates have been experimentally and numerically determined in toluene, n-pb, 1,2,4-TMB, and 1,3,5-TMB premixed flames under atmospheric pressure. The experimental results of laminar flame speed and extinction limit show that n-pb has the highest reactivity followed by toluene and TMBs. The simulation results are shown to be in reasonable agreement with the present experimental data, except that the 1,2,4-TMB model significantly underpredicts the extinction stretch rates by a maximum deviation about 40%. Sensitivity analysis of the present models demonstrated that both flame phenomena are mostly sensitive to chain-branching and heat release reactions. Further flux analysis revealed that the present models produce high reactivity of n-pb as a result of C 2 species and the accompany radicals produced during the disintegration of propyl side chain, while the high concentration of resonantly-stabled benzylic radicals is responsible for the low reactivity of TMB. Continuing efforts are underway to refine the n-pb model employed herein and develop 1,3,5-TMB model so as to encompass a larger range of experimental venues. We also note that mechanistically revealing experiments will be particularly useful in developing higher fidelity models for these alkyl aromatics
110 Laminar Flame Speed (cm/s) Laminar Flame Speed (cm/s) 70 Comparison of Laminar Flame Speeds, T u =400K Toluene 1,3,5-TMB 1,2,4-TMB n-pb Equivalence Ratio, (a) 90 Comparison of Laminar Flame Speeds, T u =470 K Toluene 1,3,5-TMB 1,2,4-TMB n-pb Equivalence Ratio, (b) Figure 6.1 Comparison of laminar flame speeds of toluene/air, n-pb/air, 1,2,4- TMB/air, and 1,3,5-TMB/air mixtures at unburned mixture temperatures of (a) T =400 K and (b) T =470 K, and pressure of P=1 atm
111 Adiabatic Flame Temperature, K Toluene n-pb 1,2,4-TMB Equivalence Ratio, Figure 6.2 Computed adiabatic flame temperatures of toluene/air, n-pb/air, and 1,2,4- TMB/air mixtures at T =400 K and P=1 atm
112 (a) (b) Figure 6.3 Pictures of near-extinction flames of n-pb/oxidizer mixtures: (a) lean flame of =0.9 (Le=2.97) and (b) rich flame of =1.2 (Le=0.95)
Confirmation of paper submission
 Dr. Marina Braun-Unkhoff Institute of Combustion Technology DLR - German Aerospace Centre Pfaffenwaldring 30-40 70569 Stuttgart 28. Mai 14 Confirmation of paper submission Name: Email: Co-author: 2nd co-author:
Dr. Marina Braun-Unkhoff Institute of Combustion Technology DLR - German Aerospace Centre Pfaffenwaldring 30-40 70569 Stuttgart 28. Mai 14 Confirmation of paper submission Name: Email: Co-author: 2nd co-author:
Foundations of Thermodynamics and Chemistry. 1 Introduction Preface Model-Building Simulation... 5 References...
 Contents Part I Foundations of Thermodynamics and Chemistry 1 Introduction... 3 1.1 Preface.... 3 1.2 Model-Building... 3 1.3 Simulation... 5 References..... 8 2 Reciprocating Engines... 9 2.1 Energy Conversion...
Contents Part I Foundations of Thermodynamics and Chemistry 1 Introduction... 3 1.1 Preface.... 3 1.2 Model-Building... 3 1.3 Simulation... 5 References..... 8 2 Reciprocating Engines... 9 2.1 Energy Conversion...
Experimental Investigation of Hot Surface Ignition of Hydrocarbon-Air Mixtures
 Paper # 2D-09 7th US National Technical Meeting of the Combustion Institute Georgia Institute of Technology, Atlanta, GA Mar 20-23, 2011. Topic: Laminar Flames Experimental Investigation of Hot Surface
Paper # 2D-09 7th US National Technical Meeting of the Combustion Institute Georgia Institute of Technology, Atlanta, GA Mar 20-23, 2011. Topic: Laminar Flames Experimental Investigation of Hot Surface
Effects of Dilution Flow Balance and Double-wall Liner on NOx Emission in Aircraft Gas Turbine Engine Combustors
 Effects of Dilution Flow Balance and Double-wall Liner on NOx Emission in Aircraft Gas Turbine Engine Combustors 9 HIDEKI MORIAI *1 Environmental regulations on aircraft, including NOx emissions, have
Effects of Dilution Flow Balance and Double-wall Liner on NOx Emission in Aircraft Gas Turbine Engine Combustors 9 HIDEKI MORIAI *1 Environmental regulations on aircraft, including NOx emissions, have
Marc ZELLAT, Driss ABOURI, Thierry CONTE and Riyad HECHAICHI CD-adapco
 16 th International Multidimensional Engine User s Meeting at the SAE Congress 2006,April,06,2006 Detroit, MI RECENT ADVANCES IN SI ENGINE MODELING: A NEW MODEL FOR SPARK AND KNOCK USING A DETAILED CHEMISTRY
16 th International Multidimensional Engine User s Meeting at the SAE Congress 2006,April,06,2006 Detroit, MI RECENT ADVANCES IN SI ENGINE MODELING: A NEW MODEL FOR SPARK AND KNOCK USING A DETAILED CHEMISTRY
Crankcase scavenging.
 Software for engine simulation and optimization www.diesel-rk.bmstu.ru The full cycle thermodynamic engine simulation software DIESEL-RK is designed for simulating and optimizing working processes of two-
Software for engine simulation and optimization www.diesel-rk.bmstu.ru The full cycle thermodynamic engine simulation software DIESEL-RK is designed for simulating and optimizing working processes of two-
Normal vs Abnormal Combustion in SI engine. SI Combustion. Turbulent Combustion
 Turbulent Combustion The motion of the charge in the engine cylinder is always turbulent, when it is reached by the flame front. The charge motion is usually composed by large vortexes, whose length scales
Turbulent Combustion The motion of the charge in the engine cylinder is always turbulent, when it is reached by the flame front. The charge motion is usually composed by large vortexes, whose length scales
Plasma Assisted Combustion in Complex Flow Environments
 High Fidelity Modeling and Simulation of Plasma Assisted Combustion in Complex Flow Environments Vigor Yang Daniel Guggenheim School of Aerospace Engineering Georgia Institute of Technology Atlanta, Georgia
High Fidelity Modeling and Simulation of Plasma Assisted Combustion in Complex Flow Environments Vigor Yang Daniel Guggenheim School of Aerospace Engineering Georgia Institute of Technology Atlanta, Georgia
COMBUSTION in SI ENGINES
 Internal Combustion Engines ME422 COMBUSTION in SI ENGINES Prof.Dr. Cem Soruşbay Internal Combustion Engines Combustion in SI Engines Introduction Classification of the combustion process Normal combustion
Internal Combustion Engines ME422 COMBUSTION in SI ENGINES Prof.Dr. Cem Soruşbay Internal Combustion Engines Combustion in SI Engines Introduction Classification of the combustion process Normal combustion
Simulation of single diesel droplet evaporation and combustion process with a unified diesel surrogate
 ILASS-Americas 29th Annual Conference on Liquid Atomization and Spray Systems, Atlanta, GA, May 2017 Simulation of single diesel droplet evaporation and combustion process with a unified diesel surrogate
ILASS-Americas 29th Annual Conference on Liquid Atomization and Spray Systems, Atlanta, GA, May 2017 Simulation of single diesel droplet evaporation and combustion process with a unified diesel surrogate
Study on cetane number dependence of. with a controlled temperature profile
 3 August 2012 (5E06) The 34th International Symposium on Combustion Study on cetane number dependence of diesel surrogates/air weak flames in a micro flow reactor with a controlled temperature profile
3 August 2012 (5E06) The 34th International Symposium on Combustion Study on cetane number dependence of diesel surrogates/air weak flames in a micro flow reactor with a controlled temperature profile
Maximizing Engine Efficiency by Controlling Fuel Reactivity Using Conventional and Alternative Fuels. Sage Kokjohn
 Maximizing Engine Efficiency by Controlling Fuel Reactivity Using Conventional and Alternative Fuels Sage Kokjohn Acknowledgments Direct-injection Engine Research Consortium (DERC) US Department of Energy/Sandia
Maximizing Engine Efficiency by Controlling Fuel Reactivity Using Conventional and Alternative Fuels Sage Kokjohn Acknowledgments Direct-injection Engine Research Consortium (DERC) US Department of Energy/Sandia
Numerical simulation of detonation inception in Hydrogen / air mixtures
 Numerical simulation of detonation inception in Hydrogen / air mixtures Ionut PORUMBEL COMOTI Non CO2 Technology Workshop, Berlin, Germany, 08.03.2017 09.03.2017 Introduction Objective: Development of
Numerical simulation of detonation inception in Hydrogen / air mixtures Ionut PORUMBEL COMOTI Non CO2 Technology Workshop, Berlin, Germany, 08.03.2017 09.03.2017 Introduction Objective: Development of
PIV ON THE FLOW IN A CATALYTIC CONVERTER
 PIV ON THE FLOW IN A CATALYTIC CONVERTER APPLICATION NOTE PIV-016 The study and optimization of the flow of exhaust through a catalytic converter is an area of research due to its potential in increasing
PIV ON THE FLOW IN A CATALYTIC CONVERTER APPLICATION NOTE PIV-016 The study and optimization of the flow of exhaust through a catalytic converter is an area of research due to its potential in increasing
Fischer-Tropsch Refining
 Fischer-Tropsch Refining by Arno de Klerk A thesis submitted in partial fulfillment of the requirements for the degree Philosophiae Doctor (Chemical Engineering) in the Department of Chemical Engineering
Fischer-Tropsch Refining by Arno de Klerk A thesis submitted in partial fulfillment of the requirements for the degree Philosophiae Doctor (Chemical Engineering) in the Department of Chemical Engineering
Homogeneous Charge Compression Ignition combustion and fuel composition
 Loughborough University Institutional Repository Homogeneous Charge Compression Ignition combustion and fuel composition This item was submitted to Loughborough University's Institutional Repository by
Loughborough University Institutional Repository Homogeneous Charge Compression Ignition combustion and fuel composition This item was submitted to Loughborough University's Institutional Repository by
Experimental Testing of a Rotating Detonation Engine Coupled to Nozzles at Conditions Approaching Flight
 25 th ICDERS August 2 7, 205 Leeds, UK Experimental Testing of a Rotating Detonation Engine Coupled to Nozzles at Conditions Approaching Flight Matthew L. Fotia*, Fred Schauer Air Force Research Laboratory
25 th ICDERS August 2 7, 205 Leeds, UK Experimental Testing of a Rotating Detonation Engine Coupled to Nozzles at Conditions Approaching Flight Matthew L. Fotia*, Fred Schauer Air Force Research Laboratory
Combustion characteristics of n-heptane droplets in a horizontal small quartz tube
 Combustion characteristics of n-heptane droplets in a horizontal small quartz tube Junwei Li*, Rong Yao, Zuozhen Qiu, Ningfei Wang School of Aerospace Engineering, Beijing Institute of Technology,Beijing
Combustion characteristics of n-heptane droplets in a horizontal small quartz tube Junwei Li*, Rong Yao, Zuozhen Qiu, Ningfei Wang School of Aerospace Engineering, Beijing Institute of Technology,Beijing
Chapter 4 ANALYTICAL WORK: COMBUSTION MODELING
 a 4.3.4 Effect of various parameters on combustion in IC engines: Compression ratio: A higher compression ratio increases the pressure and temperature of the working mixture which reduce the initial preparation
a 4.3.4 Effect of various parameters on combustion in IC engines: Compression ratio: A higher compression ratio increases the pressure and temperature of the working mixture which reduce the initial preparation
Figure 1: The spray of a direct-injecting four-stroke diesel engine
 MIXTURE FORMATION AND COMBUSTION IN CI AND SI ENGINES 7.0 Mixture Formation in Diesel Engines Diesel engines can be operated both in the two-stroke and four-stroke process. Diesel engines that run at high
MIXTURE FORMATION AND COMBUSTION IN CI AND SI ENGINES 7.0 Mixture Formation in Diesel Engines Diesel engines can be operated both in the two-stroke and four-stroke process. Diesel engines that run at high
Prediction of Physical Properties and Cetane Number of Diesel Fuels and the Effect of Aromatic Hydrocarbons on These Entities
 [Regular Paper] Prediction of Physical Properties and Cetane Number of Diesel Fuels and the Effect of Aromatic Hydrocarbons on These Entities (Received March 13, 1995) The gross heat of combustion and
[Regular Paper] Prediction of Physical Properties and Cetane Number of Diesel Fuels and the Effect of Aromatic Hydrocarbons on These Entities (Received March 13, 1995) The gross heat of combustion and
Fundamental Kinetics Database Utilizing Shock Tube Measurements
 Fundamental Kinetics Database Utilizing Shock Tube Measurements Volume 1: Ignition Delay Time Measurements D. F. Davidson and R. K. Hanson Mechanical Engineering Department Stanford University, Stanford
Fundamental Kinetics Database Utilizing Shock Tube Measurements Volume 1: Ignition Delay Time Measurements D. F. Davidson and R. K. Hanson Mechanical Engineering Department Stanford University, Stanford
Marc ZELLAT, Driss ABOURI and Stefano DURANTI CD-adapco
 17 th International Multidimensional Engine User s Meeting at the SAE Congress 2007,April,15,2007 Detroit, MI RECENT ADVANCES IN DIESEL COMBUSTION MODELING: THE ECFM- CLEH COMBUSTION MODEL: A NEW CAPABILITY
17 th International Multidimensional Engine User s Meeting at the SAE Congress 2007,April,15,2007 Detroit, MI RECENT ADVANCES IN DIESEL COMBUSTION MODELING: THE ECFM- CLEH COMBUSTION MODEL: A NEW CAPABILITY
University Turbine Systems Research Industrial Fellowship. Southwest Research Institute
 Correlating Induced Flashback with Air- Fuel Mixing Profiles for SoLoNOx Biomass Injector Ryan Ehlig University of California, Irvine Mentor: Raj Patel Supervisor: Ram Srinivasan Department Manager: Andy
Correlating Induced Flashback with Air- Fuel Mixing Profiles for SoLoNOx Biomass Injector Ryan Ehlig University of California, Irvine Mentor: Raj Patel Supervisor: Ram Srinivasan Department Manager: Andy
International Journal of Scientific & Engineering Research, Volume 5, Issue 7, July-2014 ISSN
 ISSN 9-5518 970 College of Engineering Trivandrum Department of Mechanical Engineering arundanam@gmail.com, arjunjk91@gmail.com Abstract This paper investigates the performance of a shock tube with air
ISSN 9-5518 970 College of Engineering Trivandrum Department of Mechanical Engineering arundanam@gmail.com, arjunjk91@gmail.com Abstract This paper investigates the performance of a shock tube with air
CONTROLLING COMBUSTION IN HCCI DIESEL ENGINES
 CONTROLLING COMBUSTION IN HCCI DIESEL ENGINES Nicolae Ispas *, Mircea Năstăsoiu, Mihai Dogariu Transilvania University of Brasov KEYWORDS HCCI, Diesel Engine, controlling, air-fuel mixing combustion ABSTRACT
CONTROLLING COMBUSTION IN HCCI DIESEL ENGINES Nicolae Ispas *, Mircea Năstăsoiu, Mihai Dogariu Transilvania University of Brasov KEYWORDS HCCI, Diesel Engine, controlling, air-fuel mixing combustion ABSTRACT
Experiments in a Combustion-Driven Shock Tube with an Area Change
 Accepted for presentation at the 29th International Symposium on Shock Waves. Madison, WI. July 14-19, 2013. Paper #0044 Experiments in a Combustion-Driven Shock Tube with an Area Change B. E. Schmidt
Accepted for presentation at the 29th International Symposium on Shock Waves. Madison, WI. July 14-19, 2013. Paper #0044 Experiments in a Combustion-Driven Shock Tube with an Area Change B. E. Schmidt
INVESTIGATION OF THE FUEL PROPERTY INFLUENCE ON NUMBER OF EMITTED PARTICLES AND THEIR SIZE DISTRIBUTION IN A GASOLINE ENGINE WITH DIRECT INJECTION
 INVESTIGATION OF THE FUEL PROPERTY INFLUENCE ON NUMBER OF EMITTED PARTICLES AND THEIR SIZE DISTRIBUTION IN A GASOLINE ENGINE WITH DIRECT INJECTION JAN NIKLAS GEILER 1,*, ROMAN GRZESZIK 1, THOMAS BOSSMEYER
INVESTIGATION OF THE FUEL PROPERTY INFLUENCE ON NUMBER OF EMITTED PARTICLES AND THEIR SIZE DISTRIBUTION IN A GASOLINE ENGINE WITH DIRECT INJECTION JAN NIKLAS GEILER 1,*, ROMAN GRZESZIK 1, THOMAS BOSSMEYER
Paper ID ICLASS EXPERIMENTAL INVESTIGATION OF SPRAY IMPINGEMENT ON A RAPIDLY ROTATING CYLINDER WALL
 ICLASS-26 Aug.27-Sept.1, 26, Kyoto, Japan Paper ID ICLASS6-142 EXPERIMENTAL INVESTIGATION OF SPRAY IMPINGEMENT ON A RAPIDLY ROTATING CYLINDER WALL Osman Kurt 1 and Günther Schulte 2 1 Ph.D. Student, University
ICLASS-26 Aug.27-Sept.1, 26, Kyoto, Japan Paper ID ICLASS6-142 EXPERIMENTAL INVESTIGATION OF SPRAY IMPINGEMENT ON A RAPIDLY ROTATING CYLINDER WALL Osman Kurt 1 and Günther Schulte 2 1 Ph.D. Student, University
Exhaust Gas CO vs A/F Ratio
 Title: Tuning an LPG Engine using 2-gas and 4-gas analyzers CO for Air/Fuel Ratio, and HC for Combustion Efficiency- Comparison to Lambda & Combustion Efficiency Number: 18 File:S:\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\18_CO
Title: Tuning an LPG Engine using 2-gas and 4-gas analyzers CO for Air/Fuel Ratio, and HC for Combustion Efficiency- Comparison to Lambda & Combustion Efficiency Number: 18 File:S:\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\18_CO
Combustion Equipment. Combustion equipment for. Solid fuels Liquid fuels Gaseous fuels
 Combustion Equipment Combustion equipment for Solid fuels Liquid fuels Gaseous fuels Combustion equipment Each fuel type has relative advantages and disadvantages. The same is true with regard to firing
Combustion Equipment Combustion equipment for Solid fuels Liquid fuels Gaseous fuels Combustion equipment Each fuel type has relative advantages and disadvantages. The same is true with regard to firing
HERCULES-2 Project. Deliverable: D8.8
 HERCULES-2 Project Fuel Flexible, Near Zero Emissions, Adaptive Performance Marine Engine Deliverable: D8.8 Study an alternative urea decomposition and mixer / SCR configuration and / or study in extended
HERCULES-2 Project Fuel Flexible, Near Zero Emissions, Adaptive Performance Marine Engine Deliverable: D8.8 Study an alternative urea decomposition and mixer / SCR configuration and / or study in extended
COMPUTATIONAL FLOW MODEL OF WESTFALL'S 2900 MIXER TO BE USED BY CNRL FOR BITUMEN VISCOSITY CONTROL Report R0. By Kimbal A.
 COMPUTATIONAL FLOW MODEL OF WESTFALL'S 2900 MIXER TO BE USED BY CNRL FOR BITUMEN VISCOSITY CONTROL Report 412509-1R0 By Kimbal A. Hall, PE Submitted to: WESTFALL MANUFACTURING COMPANY May 2012 ALDEN RESEARCH
COMPUTATIONAL FLOW MODEL OF WESTFALL'S 2900 MIXER TO BE USED BY CNRL FOR BITUMEN VISCOSITY CONTROL Report 412509-1R0 By Kimbal A. Hall, PE Submitted to: WESTFALL MANUFACTURING COMPANY May 2012 ALDEN RESEARCH
Module 2:Genesis and Mechanism of Formation of Engine Emissions Lecture 9:Mechanisms of HC Formation in SI Engines... contd.
 Mechanisms of HC Formation in SI Engines... contd. The Lecture Contains: HC from Lubricating Oil Film Combustion Chamber Deposits HC Mixture Quality and In-Cylinder Liquid Fuel HC from Misfired Combustion
Mechanisms of HC Formation in SI Engines... contd. The Lecture Contains: HC from Lubricating Oil Film Combustion Chamber Deposits HC Mixture Quality and In-Cylinder Liquid Fuel HC from Misfired Combustion
Auto-ignition of Premixed Methane/air Mixture in the Presence of Dust
 25 th ICDERS August 2 7, 2015 Leeds, UK Auto-ignition of Premixed Methane/air Mixture in the Presence of Dust V.V. Leschevich, O.G. Penyazkov, S.Yu. Shimchenko Physical and Chemical Hydrodynamics Laboratory,
25 th ICDERS August 2 7, 2015 Leeds, UK Auto-ignition of Premixed Methane/air Mixture in the Presence of Dust V.V. Leschevich, O.G. Penyazkov, S.Yu. Shimchenko Physical and Chemical Hydrodynamics Laboratory,
Recent enhancement to SI-ICE combustion models: Application to stratified combustion under large EGR rate and lean burn
 Recent enhancement to SI-ICE combustion models: Application to stratified combustion under large EGR rate and lean burn G. Desoutter, A. Desportes, J. Hira, D. Abouri, K.Oberhumer, M. Zellat* TOPICS Introduction
Recent enhancement to SI-ICE combustion models: Application to stratified combustion under large EGR rate and lean burn G. Desoutter, A. Desportes, J. Hira, D. Abouri, K.Oberhumer, M. Zellat* TOPICS Introduction
White Paper. Improving Accuracy and Precision in Crude Oil Boiling Point Distribution Analysis. Introduction. Background Information
 Improving Accuracy and Precision in Crude Oil Boiling Point Distribution Analysis. Abstract High Temperature Simulated Distillation (High Temp SIMDIS) is one of the most frequently used techniques to determine
Improving Accuracy and Precision in Crude Oil Boiling Point Distribution Analysis. Abstract High Temperature Simulated Distillation (High Temp SIMDIS) is one of the most frequently used techniques to determine
Overview & Perspectives for Internal Combustion Engine using STAR-CD. Marc ZELLAT
 Overview & Perspectives for Internal Combustion Engine using STAR-CD Marc ZELLAT TOPICS Quick overview of ECFM family models Examples of validation for Diesel and SI-GDI engines Introduction to multi-component
Overview & Perspectives for Internal Combustion Engine using STAR-CD Marc ZELLAT TOPICS Quick overview of ECFM family models Examples of validation for Diesel and SI-GDI engines Introduction to multi-component
Development of Low-Exergy-Loss, High-Efficiency Chemical Engines
 Development of Low-Exergy-Loss, High-Efficiency Chemical Engines Investigators C. F., Associate Professor, Mechanical Engineering; Kwee-Yan Teh, Shannon L. Miller, Graduate Researchers Introduction The
Development of Low-Exergy-Loss, High-Efficiency Chemical Engines Investigators C. F., Associate Professor, Mechanical Engineering; Kwee-Yan Teh, Shannon L. Miller, Graduate Researchers Introduction The
COMBUSTION in SI ENGINES
 Internal Combustion Engines MAK 493E COMBUSTION in SI ENGINES Prof.Dr. Cem Soruşbay Istanbul Technical University Internal Combustion Engines MAK 493E Combustion in SI Engines Introduction Classification
Internal Combustion Engines MAK 493E COMBUSTION in SI ENGINES Prof.Dr. Cem Soruşbay Istanbul Technical University Internal Combustion Engines MAK 493E Combustion in SI Engines Introduction Classification
POSIBILITIES TO IMPROVED HOMOGENEOUS CHARGE IN INTERNAL COMBUSTION ENGINES, USING C.F.D. PROGRAM
 POSIBILITIES TO IMPROVED HOMOGENEOUS CHARGE IN INTERNAL COMBUSTION ENGINES, USING C.F.D. PROGRAM Alexandru-Bogdan Muntean *, Anghel,Chiru, Ruxandra-Cristina (Dica) Stanescu, Cristian Soimaru Transilvania
POSIBILITIES TO IMPROVED HOMOGENEOUS CHARGE IN INTERNAL COMBUSTION ENGINES, USING C.F.D. PROGRAM Alexandru-Bogdan Muntean *, Anghel,Chiru, Ruxandra-Cristina (Dica) Stanescu, Cristian Soimaru Transilvania
Master of Engineering
 STUDIES OF FAULT CURRENT LIMITERS FOR POWER SYSTEMS PROTECTION A Project Report Submitted in partial fulfilment of the requirements for the Degree of Master of Engineering In INFORMATION AND TELECOMMUNICATION
STUDIES OF FAULT CURRENT LIMITERS FOR POWER SYSTEMS PROTECTION A Project Report Submitted in partial fulfilment of the requirements for the Degree of Master of Engineering In INFORMATION AND TELECOMMUNICATION
Institut für Thermische Strömungsmaschinen. PDA Measurements of the Stationary Reacting Flow
 Institut für Thermische Strömungsmaschinen Dr.-Ing. Rainer Koch Dipl.-Ing. Tamas Laza DELIVERABLE D2.2 PDA Measurements of the Stationary Reacting Flow CONTRACT N : PROJECT N : ACRONYM: TITLE: TASK 2.1:
Institut für Thermische Strömungsmaschinen Dr.-Ing. Rainer Koch Dipl.-Ing. Tamas Laza DELIVERABLE D2.2 PDA Measurements of the Stationary Reacting Flow CONTRACT N : PROJECT N : ACRONYM: TITLE: TASK 2.1:
Recent Advances in DI-Diesel Combustion Modeling in AVL FIRE A Validation Study
 International Multidimensional Engine Modeling User s Group Meeting at the SAE Congress April 15, 2007 Detroit, MI Recent Advances in DI-Diesel Combustion Modeling in AVL FIRE A Validation Study R. Tatschl,
International Multidimensional Engine Modeling User s Group Meeting at the SAE Congress April 15, 2007 Detroit, MI Recent Advances in DI-Diesel Combustion Modeling in AVL FIRE A Validation Study R. Tatschl,
R&D on a Medium-speed, Four-cycle Diesel Engine Using Heavy fuel oil
 1999C.4.1.11 R&D on a Medium-speed, Four-cycle Diesel Engine Using Heavy fuel oil 1. R&D contents 1.1 Background and R&D objectives In order to meet increasing demand for light oil and intermediate fraction,
1999C.4.1.11 R&D on a Medium-speed, Four-cycle Diesel Engine Using Heavy fuel oil 1. R&D contents 1.1 Background and R&D objectives In order to meet increasing demand for light oil and intermediate fraction,
CHAPTER 1 INTRODUCTION
 1 CHAPTER 1 INTRODUCTION 1.1 GENERAL Diesel engines are the primary power source of vehicles used in heavy duty applications. The heavy duty engine includes buses, large trucks, and off-highway construction
1 CHAPTER 1 INTRODUCTION 1.1 GENERAL Diesel engines are the primary power source of vehicles used in heavy duty applications. The heavy duty engine includes buses, large trucks, and off-highway construction
Combustion Properties of Alternative Liquid Fuels
 1. Prologue Combustion Properties of Alternative Liquid Fuels 21 JULY 211 Cheng Tung Chong, Simone Hochgreb Content 1. Introduction 2. What s biodiesels 3. Burner design and experimental 4. Results - Flame
1. Prologue Combustion Properties of Alternative Liquid Fuels 21 JULY 211 Cheng Tung Chong, Simone Hochgreb Content 1. Introduction 2. What s biodiesels 3. Burner design and experimental 4. Results - Flame
EFFECT OF INJECTION ORIENTATION ON EXHAUST EMISSIONS IN A DI DIESEL ENGINE: THROUGH CFD SIMULATION
 EFFECT OF INJECTION ORIENTATION ON EXHAUST EMISSIONS IN A DI DIESEL ENGINE: THROUGH CFD SIMULATION *P. Manoj Kumar 1, V. Pandurangadu 2, V.V. Pratibha Bharathi 3 and V.V. Naga Deepthi 4 1 Department of
EFFECT OF INJECTION ORIENTATION ON EXHAUST EMISSIONS IN A DI DIESEL ENGINE: THROUGH CFD SIMULATION *P. Manoj Kumar 1, V. Pandurangadu 2, V.V. Pratibha Bharathi 3 and V.V. Naga Deepthi 4 1 Department of
5. Combustion of liquid fuels. 5.1 Atomization of fuel
 5. Combustion of liquid fuels 5.1 Atomization of fuel iquid fuels such as gasoline, diesel, fuel oil light, fuel oil heavy or kerosene have to be atomized and well mixed with the combustion air before
5. Combustion of liquid fuels 5.1 Atomization of fuel iquid fuels such as gasoline, diesel, fuel oil light, fuel oil heavy or kerosene have to be atomized and well mixed with the combustion air before
Module 3: Influence of Engine Design and Operating Parameters on Emissions Lecture 14:Effect of SI Engine Design and Operating Variables on Emissions
 Module 3: Influence of Engine Design and Operating Parameters on Emissions Effect of SI Engine Design and Operating Variables on Emissions The Lecture Contains: SI Engine Variables and Emissions Compression
Module 3: Influence of Engine Design and Operating Parameters on Emissions Effect of SI Engine Design and Operating Variables on Emissions The Lecture Contains: SI Engine Variables and Emissions Compression
Module7:Advanced Combustion Systems and Alternative Powerplants Lecture 32:Stratified Charge Engines
 ADVANCED COMBUSTION SYSTEMS AND ALTERNATIVE POWERPLANTS The Lecture Contains: DIRECT INJECTION STRATIFIED CHARGE (DISC) ENGINES Historical Overview Potential Advantages of DISC Engines DISC Engine Combustion
ADVANCED COMBUSTION SYSTEMS AND ALTERNATIVE POWERPLANTS The Lecture Contains: DIRECT INJECTION STRATIFIED CHARGE (DISC) ENGINES Historical Overview Potential Advantages of DISC Engines DISC Engine Combustion
High Pressure Spray Characterization of Vegetable Oils
 , 23rd Annual Conference on Liquid Atomization and Spray Systems, Brno, Czech Republic, September 2010 Devendra Deshmukh, A. Madan Mohan, T. N. C. Anand and R. V. Ravikrishna Department of Mechanical Engineering
, 23rd Annual Conference on Liquid Atomization and Spray Systems, Brno, Czech Republic, September 2010 Devendra Deshmukh, A. Madan Mohan, T. N. C. Anand and R. V. Ravikrishna Department of Mechanical Engineering
Lecture 27: Principles of Burner Design
 Lecture 27: Principles of Burner Design Contents: How does combustion occur? What is a burner? Mixing of air and gaseous fuel Characteristic features of jet Behavior of free (unconfined) and confined jet
Lecture 27: Principles of Burner Design Contents: How does combustion occur? What is a burner? Mixing of air and gaseous fuel Characteristic features of jet Behavior of free (unconfined) and confined jet
Perfectly Stirred Reactor Network Modeling of NOx and CO Emissions from a Gas Turbine Combustor with Water Addition
 Perfectly Stirred Reactor Network Modeling of NOx and CO Emissions from a Gas Turbine Combustor with Water Addition Abstract For Submission in Partial Fulfillment of the UTSR Fellowship Program Andrew
Perfectly Stirred Reactor Network Modeling of NOx and CO Emissions from a Gas Turbine Combustor with Water Addition Abstract For Submission in Partial Fulfillment of the UTSR Fellowship Program Andrew
METHANE/OXYGEN LASER IGNITION IN AN EXPERIMENTAL ROCKET COMBUSTION CHAMBER: IMPACT OF MIXING AND IGNITION POSITION
 SP2016_3124927 METHANE/OXYGEN LASER IGNITION IN AN EXPERIMENTAL ROCKET COMBUSTION CHAMBER: IMPACT OF MIXING AND IGNITION POSITION Michael Wohlhüter, Victor P. Zhukov, Michael Börner Institute of Space
SP2016_3124927 METHANE/OXYGEN LASER IGNITION IN AN EXPERIMENTAL ROCKET COMBUSTION CHAMBER: IMPACT OF MIXING AND IGNITION POSITION Michael Wohlhüter, Victor P. Zhukov, Michael Börner Institute of Space
PDF-based simulations of in-cylinder combustion in a compression-ignition engine
 Paper # 070IC-0192 Topic: Internal Combustion Engines 8 th US National Combustion Meeting Organized by the Western States Section of the Combustion Institute and hosted by the University of Utah May 19-22,
Paper # 070IC-0192 Topic: Internal Combustion Engines 8 th US National Combustion Meeting Organized by the Western States Section of the Combustion Institute and hosted by the University of Utah May 19-22,
Oil & Gas. From exploration to distribution. Week 3 V19 Refining Processes (Part 1) Jean-Luc Monsavoir. W3V19 - Refining Processes1 p.
 Oil & Gas From exploration to distribution Week 3 V19 Refining Processes (Part 1) Jean-Luc Monsavoir W3V19 - Refining Processes1 p. 1 Crude Oil Origins and Composition The objective of refining, petrochemical
Oil & Gas From exploration to distribution Week 3 V19 Refining Processes (Part 1) Jean-Luc Monsavoir W3V19 - Refining Processes1 p. 1 Crude Oil Origins and Composition The objective of refining, petrochemical
Zürich Testing on Fuel Effects and Future Work Programme
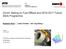 Zürich Testing on Fuel Effects and 2016-2017 Future Work Programme Benjamin Brem 1,2, Lukas Durdina 1,2 and Jing Wang 1,2 1 Empa 2 ETH Zürich FORUM on Aviation and Emissions Workshop Amsterdam 15.04.2016
Zürich Testing on Fuel Effects and 2016-2017 Future Work Programme Benjamin Brem 1,2, Lukas Durdina 1,2 and Jing Wang 1,2 1 Empa 2 ETH Zürich FORUM on Aviation and Emissions Workshop Amsterdam 15.04.2016
CHAPTER 8 EFFECTS OF COMBUSTION CHAMBER GEOMETRIES
 112 CHAPTER 8 EFFECTS OF COMBUSTION CHAMBER GEOMETRIES 8.1 INTRODUCTION Energy conservation and emissions have become of increasing concern over the past few decades. More stringent emission laws along
112 CHAPTER 8 EFFECTS OF COMBUSTION CHAMBER GEOMETRIES 8.1 INTRODUCTION Energy conservation and emissions have become of increasing concern over the past few decades. More stringent emission laws along
SWIRL MEASURING EQUIPMENT FOR DIRECT INJECTION DIESEL ENGINE
 SWIRL MEASURING EQUIPMENT FOR DIRECT INJECTION DIESEL ENGINE G.S.Gosavi 1, R.B.Solankar 2, A.R.Kori 3, R.B.Chavan 4, S.P.Shinde 5 1,2,3,4,5 Mechanical Engineering Department, Shivaji University, (India)
SWIRL MEASURING EQUIPMENT FOR DIRECT INJECTION DIESEL ENGINE G.S.Gosavi 1, R.B.Solankar 2, A.R.Kori 3, R.B.Chavan 4, S.P.Shinde 5 1,2,3,4,5 Mechanical Engineering Department, Shivaji University, (India)
CHEMSYSTEMS. Report Abstract. Petrochemical Market Dynamics Feedstocks
 CHEMSYSTEMS PPE PROGRAM Report Abstract Petrochemical Market Dynamics Feedstocks Petrochemical feedstocks industry overview, crude oil, natural gas, coal, biological hydrocarbons, olefins, aromatics, methane
CHEMSYSTEMS PPE PROGRAM Report Abstract Petrochemical Market Dynamics Feedstocks Petrochemical feedstocks industry overview, crude oil, natural gas, coal, biological hydrocarbons, olefins, aromatics, methane
Chapter 5 Oxygen Based NOx Control
 Chapter 5 Oxygen Based NOx Control Editor s Note: Chapter 5 is written by Dr. Brian Doyle and is drawn primarily from personal knowledge and the material developed for the NOx Emissions course offered
Chapter 5 Oxygen Based NOx Control Editor s Note: Chapter 5 is written by Dr. Brian Doyle and is drawn primarily from personal knowledge and the material developed for the NOx Emissions course offered
Experimental Verification of Low Emission Combustor Technology at DLR
 www.dlr.de Chart 1 > FORUM-AE Non-CO2 mitigation technology Workshop> Hassa > 2.7.2014 Experimental Verification of Low Emission Combustor Technology at DLR Christoph Hassa Institute of Propulsion Technology
www.dlr.de Chart 1 > FORUM-AE Non-CO2 mitigation technology Workshop> Hassa > 2.7.2014 Experimental Verification of Low Emission Combustor Technology at DLR Christoph Hassa Institute of Propulsion Technology
Optical Techniques in Gasoline Engine Performance and Emissions Development Injector Spray Visualisation
 Injector Spray Visualisation Denis Gill, Wolfgang Krankenedl, DEC Ernst Winklhofer 20.03.15 Emissions Development Injector Spray Visualisation Contents Introduction Spray Box Direct Injection (GDI) Spray
Injector Spray Visualisation Denis Gill, Wolfgang Krankenedl, DEC Ernst Winklhofer 20.03.15 Emissions Development Injector Spray Visualisation Contents Introduction Spray Box Direct Injection (GDI) Spray
Structural Analysis Of Reciprocating Compressor Manifold
 Purdue University Purdue e-pubs International Compressor Engineering Conference School of Mechanical Engineering 2016 Structural Analysis Of Reciprocating Compressor Manifold Marcos Giovani Dropa Bortoli
Purdue University Purdue e-pubs International Compressor Engineering Conference School of Mechanical Engineering 2016 Structural Analysis Of Reciprocating Compressor Manifold Marcos Giovani Dropa Bortoli
ACTUAL CYCLE. Actual engine cycle
 1 ACTUAL CYCLE Actual engine cycle Introduction 2 Ideal Gas Cycle (Air Standard Cycle) Idealized processes Idealize working Fluid Fuel-Air Cycle Idealized Processes Accurate Working Fluid Model Actual
1 ACTUAL CYCLE Actual engine cycle Introduction 2 Ideal Gas Cycle (Air Standard Cycle) Idealized processes Idealize working Fluid Fuel-Air Cycle Idealized Processes Accurate Working Fluid Model Actual
1. INTRODUCTION 2. EXPERIMENTAL INVESTIGATIONS
 HIGH PRESSURE HYDROGEN INJECTION SYSTEM FOR A LARGE BORE 4 STROKE DIESEL ENGINE: INVESTIGATION OF THE MIXTURE FORMATION WITH LASER-OPTICAL MEASUREMENT TECHNIQUES AND NUMERICAL SIMULATIONS Dipl.-Ing. F.
HIGH PRESSURE HYDROGEN INJECTION SYSTEM FOR A LARGE BORE 4 STROKE DIESEL ENGINE: INVESTIGATION OF THE MIXTURE FORMATION WITH LASER-OPTICAL MEASUREMENT TECHNIQUES AND NUMERICAL SIMULATIONS Dipl.-Ing. F.
MODELING AND ANALYSIS OF DIESEL ENGINE WITH ADDITION OF HYDROGEN-HYDROGEN-OXYGEN GAS
 S465 MODELING AND ANALYSIS OF DIESEL ENGINE WITH ADDITION OF HYDROGEN-HYDROGEN-OXYGEN GAS by Karu RAGUPATHY* Department of Automobile Engineering, Dr. Mahalingam College of Engineering and Technology,
S465 MODELING AND ANALYSIS OF DIESEL ENGINE WITH ADDITION OF HYDROGEN-HYDROGEN-OXYGEN GAS by Karu RAGUPATHY* Department of Automobile Engineering, Dr. Mahalingam College of Engineering and Technology,
Proposal to establish a laboratory for combustion studies
 Proposal to establish a laboratory for combustion studies Jayr de Amorim Filho Brazilian Bioethanol Science and Technology Laboratory SCRE Single Cylinder Research Engine Laboratory OUTLINE Requirements,
Proposal to establish a laboratory for combustion studies Jayr de Amorim Filho Brazilian Bioethanol Science and Technology Laboratory SCRE Single Cylinder Research Engine Laboratory OUTLINE Requirements,
Investigation of a promising method for liquid hydrocarbons spraying
 Journal of Physics: Conference Series PAPER OPEN ACCESS Investigation of a promising method for liquid hydrocarbons spraying To cite this article: E P Kopyev and E Yu Shadrin 2018 J. Phys.: Conf. Ser.
Journal of Physics: Conference Series PAPER OPEN ACCESS Investigation of a promising method for liquid hydrocarbons spraying To cite this article: E P Kopyev and E Yu Shadrin 2018 J. Phys.: Conf. Ser.
PERFORMANCE ESTIMATION AND ANALYSIS OF PULSE DETONATION ENGINE WITH DIFFERENT BLOCKAGE RATIOS FOR HYDROGEN-AIR MIXTURE
 PERFORMANCE ESTIMATION AND ANALYSIS OF PULSE DETONATION ENGINE WITH DIFFERENT BLOCKAGE RATIOS FOR HYDROGEN-AIR MIXTURE Nadella Karthik 1, Repaka Ramesh 2, N.V.V.K Chaitanya 3, Linsu Sebastian 4 1,2,3,4
PERFORMANCE ESTIMATION AND ANALYSIS OF PULSE DETONATION ENGINE WITH DIFFERENT BLOCKAGE RATIOS FOR HYDROGEN-AIR MIXTURE Nadella Karthik 1, Repaka Ramesh 2, N.V.V.K Chaitanya 3, Linsu Sebastian 4 1,2,3,4
Experimental Investigation of Flame Speed of Gasoline Fuel-Air Mixture
 International Journal of Current Engineering and Technology E-ISSN 2277 4106, P-ISSN 2347 5161 2018 INPRESSCO, All Rights Reserved Available at http://inpressco.com/category/ijcet Research Article Experimental
International Journal of Current Engineering and Technology E-ISSN 2277 4106, P-ISSN 2347 5161 2018 INPRESSCO, All Rights Reserved Available at http://inpressco.com/category/ijcet Research Article Experimental
Gas exchange process for IC-engines: poppet valves, valve timing and variable valve actuation
 Gas exchange process for IC-engines: poppet valves, valve timing and variable valve actuation Topics Analysis of the main parameters influencing the volumetric efficiency in IC engines: - Valves and valve
Gas exchange process for IC-engines: poppet valves, valve timing and variable valve actuation Topics Analysis of the main parameters influencing the volumetric efficiency in IC engines: - Valves and valve
Shock-tube study of the addition effect of CF 2 BrCl on the ignition of light hydrocarbons
 25 th ICDERS August 2 7, 2015 Leeds, UK Shock-tube study of the addition effect of CF 2 BrCl on the ignition of light hydrocarbons O. Mathieu, C. Gregoire, and E. L. Petersen Texas A&M University, Department
25 th ICDERS August 2 7, 2015 Leeds, UK Shock-tube study of the addition effect of CF 2 BrCl on the ignition of light hydrocarbons O. Mathieu, C. Gregoire, and E. L. Petersen Texas A&M University, Department
The Effect of Volume Ratio of Ethanol Directly Injected in a Gasoline Port Injection Spark Ignition Engine
 10 th ASPACC July 19 22, 2015 Beijing, China The Effect of Volume Ratio of Ethanol Directly Injected in a Gasoline Port Injection Spark Ignition Engine Yuhan Huang a,b, Guang Hong a, Ronghua Huang b. a
10 th ASPACC July 19 22, 2015 Beijing, China The Effect of Volume Ratio of Ethanol Directly Injected in a Gasoline Port Injection Spark Ignition Engine Yuhan Huang a,b, Guang Hong a, Ronghua Huang b. a
INFLUENCE OF THE NUMBER OF NOZZLE HOLES ON THE UNBURNED FUEL IN DIESEL ENGINE
 INFLUENCE OF THE NUMBER OF NOZZLE HOLES ON THE UNBURNED FUEL IN DIESEL ENGINE 1. UNIVERSITY OF RUSE, 8, STUDENTSKA STR., 7017 RUSE, BULGARIA 1. Simeon ILIEV ABSTRACT: The objective of this paper is to
INFLUENCE OF THE NUMBER OF NOZZLE HOLES ON THE UNBURNED FUEL IN DIESEL ENGINE 1. UNIVERSITY OF RUSE, 8, STUDENTSKA STR., 7017 RUSE, BULGARIA 1. Simeon ILIEV ABSTRACT: The objective of this paper is to
NUMERICAL INVESTIGATION OF PISTON COOLING USING SINGLE CIRCULAR OIL JET IMPINGEMENT
 NUMERICAL INVESTIGATION OF PISTON COOLING USING SINGLE CIRCULAR OIL JET IMPINGEMENT BALAKRISHNAN RAJU, CFD ANALYSIS ENGINEER, TATA CONSULTANCY SERVICES LTD., BANGALORE ABSTRACT Thermal loading of piston
NUMERICAL INVESTIGATION OF PISTON COOLING USING SINGLE CIRCULAR OIL JET IMPINGEMENT BALAKRISHNAN RAJU, CFD ANALYSIS ENGINEER, TATA CONSULTANCY SERVICES LTD., BANGALORE ABSTRACT Thermal loading of piston
Emissions predictions for Diesel engines based on chemistry tabulation
 Emissions predictions for Diesel engines based on chemistry tabulation C. Meijer, F.A. Tap AVL Dacolt BV (The Netherlands) M. Tvrdojevic, P. Priesching AVL List GmbH (Austria) 1. Introduction It is generally
Emissions predictions for Diesel engines based on chemistry tabulation C. Meijer, F.A. Tap AVL Dacolt BV (The Netherlands) M. Tvrdojevic, P. Priesching AVL List GmbH (Austria) 1. Introduction It is generally
Investigation of converging slot-hole geometry for film cooling of gas turbine blades
 Project Report 2010 MVK160 Heat and Mass Transport May 12, 2010, Lund, Sweden Investigation of converging slot-hole geometry for film cooling of gas turbine blades Tobias Pihlstrand Dept. of Energy Sciences,
Project Report 2010 MVK160 Heat and Mass Transport May 12, 2010, Lund, Sweden Investigation of converging slot-hole geometry for film cooling of gas turbine blades Tobias Pihlstrand Dept. of Energy Sciences,
Fuel Related Definitions
 Fuel Related Definitions ASH The solid residue left when combustible material is thoroughly burned or is oxidized by chemical means. The ash content of a fuel is the non combustible residue found in the
Fuel Related Definitions ASH The solid residue left when combustible material is thoroughly burned or is oxidized by chemical means. The ash content of a fuel is the non combustible residue found in the
Theoretical Study of the effects of Ignition Delay on the Performance of DI Diesel Engine
 Theoretical Study of the effects of Ignition Delay on the Performance of DI Diesel Engine Vivek Shankhdhar a, Neeraj Kumar b a M.Tech Scholar, Moradabad Institute of Technology, India b Asst. Proff. Mechanical
Theoretical Study of the effects of Ignition Delay on the Performance of DI Diesel Engine Vivek Shankhdhar a, Neeraj Kumar b a M.Tech Scholar, Moradabad Institute of Technology, India b Asst. Proff. Mechanical
Available online at ScienceDirect. Procedia CIRP 33 (2015 )
 Available online at www.sciencedirect.com ScienceDirect Procedia CIRP 33 (2015 ) 581 586 9th CIRP Conference on Intelligent Computation in Manufacturing Engineering - CIRP ICME '14 Magnetic fluid seal
Available online at www.sciencedirect.com ScienceDirect Procedia CIRP 33 (2015 ) 581 586 9th CIRP Conference on Intelligent Computation in Manufacturing Engineering - CIRP ICME '14 Magnetic fluid seal
Detection of Volatile Organic Compounds in Gasoline and Diesel Using the znose Edward J. Staples, Electronic Sensor Technology
 Detection of Volatile Organic Compounds in Gasoline and Diesel Using the znose Edward J. Staples, Electronic Sensor Technology Electronic Noses An electronic nose produces a recognizable response based
Detection of Volatile Organic Compounds in Gasoline and Diesel Using the znose Edward J. Staples, Electronic Sensor Technology Electronic Noses An electronic nose produces a recognizable response based
Optimizing Combustion Processes. Facilitating Cost-effective and Environmentally Friendly Products
 Optimizing Combustion Processes Facilitating Cost-effective and Environmentally Friendly Products The combustion process poses significant challenges with respect to maximizing performance and fuel economy.
Optimizing Combustion Processes Facilitating Cost-effective and Environmentally Friendly Products The combustion process poses significant challenges with respect to maximizing performance and fuel economy.
Cavitating Flow in a Model Diesel Injector Return Valve
 Introduction Cavitating Flow in a Model Diesel Injector Return Valve 1 Alberto Bonifacio; 1 Russel Lockett*, Richard Price 1 Department of Mechanical Engineering & Aeronautics, City, University of London,
Introduction Cavitating Flow in a Model Diesel Injector Return Valve 1 Alberto Bonifacio; 1 Russel Lockett*, Richard Price 1 Department of Mechanical Engineering & Aeronautics, City, University of London,
AUTOMOTIVE TESTING AND OPTIMIZATION. Tools for designing tomorrow's vehicles
 AUTOMOTIVE TESTING AND OPTIMIZATION Tools for designing tomorrow's vehicles 2 Measurement of flow around the side mirror by Particle Image Velocimetry (PIV). Courtesy of Visteon Deutschland GmbH Our advanced
AUTOMOTIVE TESTING AND OPTIMIZATION Tools for designing tomorrow's vehicles 2 Measurement of flow around the side mirror by Particle Image Velocimetry (PIV). Courtesy of Visteon Deutschland GmbH Our advanced
DARS FUEL MODEL DEVELOPMENT
 DARS FUEL MODEL DEVELOPMENT DARS Products (names valid since October 2012) DARS 0D & 1D tools Old name: DARS Basic DARS Reactive Flow Models tools for 3D/ CFD calculations DARS Fuel New! Advanced fuel
DARS FUEL MODEL DEVELOPMENT DARS Products (names valid since October 2012) DARS 0D & 1D tools Old name: DARS Basic DARS Reactive Flow Models tools for 3D/ CFD calculations DARS Fuel New! Advanced fuel
A Study of EGR Stratification in an Engine Cylinder
 A Study of EGR Stratification in an Engine Cylinder Bassem Ramadan Kettering University ABSTRACT One strategy to decrease the amount of oxides of nitrogen formed and emitted from certain combustion devices,
A Study of EGR Stratification in an Engine Cylinder Bassem Ramadan Kettering University ABSTRACT One strategy to decrease the amount of oxides of nitrogen formed and emitted from certain combustion devices,
Presenter: Sébastien Bourgois (SN)
 Multi point i injection i system development at Snecma Presenter: Sébastien Bourgois (SN) Outline Overview of Multipoint Injection System development at SNECMA Tools used for conception An example: LEMCOTEC
Multi point i injection i system development at Snecma Presenter: Sébastien Bourgois (SN) Outline Overview of Multipoint Injection System development at SNECMA Tools used for conception An example: LEMCOTEC
Application of ABAQUS to Analyzing Shrink Fitting Process of Semi Built-up Type Marine Engine Crankshaft
 Application of ABAQUS to Analyzing Shrink Fitting Process of Semi Built-up Type Marine Engine Crankshaft Jae-Cheol Kim, Dong-Kwon Kim, Young-Duk Kim, and Dong-Young Kim System Technology Research Team,
Application of ABAQUS to Analyzing Shrink Fitting Process of Semi Built-up Type Marine Engine Crankshaft Jae-Cheol Kim, Dong-Kwon Kim, Young-Duk Kim, and Dong-Young Kim System Technology Research Team,
Progress in Predicting Soot Particle Numbers in CFD Simulations of GDI and Diesel Engines
 International Multidimensional Engine Modeling User's Group Meeting April 20, 2015, Detroit, Michigan Progress in Predicting Soot Particle Numbers in CFD Simulations of GDI and Diesel Engines Abstract
International Multidimensional Engine Modeling User's Group Meeting April 20, 2015, Detroit, Michigan Progress in Predicting Soot Particle Numbers in CFD Simulations of GDI and Diesel Engines Abstract
PERFORMANCE AND EMISSION ANALYSIS OF DIESEL ENGINE BY INJECTING DIETHYL ETHER WITH AND WITHOUT EGR USING DPF
 PERFORMANCE AND EMISSION ANALYSIS OF DIESEL ENGINE BY INJECTING DIETHYL ETHER WITH AND WITHOUT EGR USING DPF PROJECT REFERENCE NO. : 37S1036 COLLEGE BRANCH GUIDES : KS INSTITUTE OF TECHNOLOGY, BANGALORE
PERFORMANCE AND EMISSION ANALYSIS OF DIESEL ENGINE BY INJECTING DIETHYL ETHER WITH AND WITHOUT EGR USING DPF PROJECT REFERENCE NO. : 37S1036 COLLEGE BRANCH GUIDES : KS INSTITUTE OF TECHNOLOGY, BANGALORE
Experimental Study of LPG Diffusion Flame at Elevated Preheated Air Temperatures
 Experimental Study of LPG Diffusion Flame at Elevated Preheated Air Temperatures A. A. Amer, H. M. Gad, I. A. Ibrahim, S. I. Abdel-Mageed, T. M. Farag Abstract This paper represents an experimental study
Experimental Study of LPG Diffusion Flame at Elevated Preheated Air Temperatures A. A. Amer, H. M. Gad, I. A. Ibrahim, S. I. Abdel-Mageed, T. M. Farag Abstract This paper represents an experimental study
Dual Fuel Engine Charge Motion & Combustion Study
 Dual Fuel Engine Charge Motion & Combustion Study STAR-Global-Conference March 06-08, 2017 Berlin Kamlesh Ghael, Prof. Dr. Sebastian Kaiser (IVG-RF), M. Sc. Felix Rosenthal (IFKM-KIT) Introduction: Operation
Dual Fuel Engine Charge Motion & Combustion Study STAR-Global-Conference March 06-08, 2017 Berlin Kamlesh Ghael, Prof. Dr. Sebastian Kaiser (IVG-RF), M. Sc. Felix Rosenthal (IFKM-KIT) Introduction: Operation
CFD Simulation of Dry Low Nox Turbogas Combustion System
 CFD Simulation of Dry Low Nox Turbogas Combustion System L. Bucchieri - Engin Soft F. Turrini - Fiat Avio CFX Users Conference - Friedrichshafen June 1999 1 Objectives Develop a CFD model for turbogas
CFD Simulation of Dry Low Nox Turbogas Combustion System L. Bucchieri - Engin Soft F. Turrini - Fiat Avio CFX Users Conference - Friedrichshafen June 1999 1 Objectives Develop a CFD model for turbogas
This presentation focuses on Biodiesel, scientifically called FAME (Fatty Acid Methyl Ester); a fuel different in either perspective.
 Today, we know a huge variety of so-called alternative fuels which are usually regarded as biofuels, even though this is not always true. Alternative fuels can replace fossil fuels in existing combustion
Today, we know a huge variety of so-called alternative fuels which are usually regarded as biofuels, even though this is not always true. Alternative fuels can replace fossil fuels in existing combustion
Modelling Combustion in DI-SI using the G-equation Method and Detailed Chemistry: Emissions and knock. M.Zellat, D.Abouri, Y.Liang, C.
 Modelling Combustion in DI-SI using the G-equation Method and Detailed Chemistry: Emissions and knock Realize innovation. M.Zellat, D.Abouri, Y.Liang, C.Kralj Main topics of the presentation 1. Context
Modelling Combustion in DI-SI using the G-equation Method and Detailed Chemistry: Emissions and knock Realize innovation. M.Zellat, D.Abouri, Y.Liang, C.Kralj Main topics of the presentation 1. Context
Article: The Formation & Testing of Sludge in Bunker Fuels By Dr Sunil Kumar Laboratory Manager VPS Fujairah 15th January 2018
 Article: The Formation & Testing of Sludge in Bunker Fuels By Dr Sunil Kumar Laboratory Manager VPS Fujairah 15th January 2018 Introduction Sludge formation in bunker fuel is the source of major operational
Article: The Formation & Testing of Sludge in Bunker Fuels By Dr Sunil Kumar Laboratory Manager VPS Fujairah 15th January 2018 Introduction Sludge formation in bunker fuel is the source of major operational
Module 2:Genesis and Mechanism of Formation of Engine Emissions Lecture 3: Introduction to Pollutant Formation POLLUTANT FORMATION
 Module 2:Genesis and Mechanism of Formation of Engine Emissions POLLUTANT FORMATION The Lecture Contains: Engine Emissions Typical Exhaust Emission Concentrations Emission Formation in SI Engines Emission
Module 2:Genesis and Mechanism of Formation of Engine Emissions POLLUTANT FORMATION The Lecture Contains: Engine Emissions Typical Exhaust Emission Concentrations Emission Formation in SI Engines Emission
Module8:Engine Fuels and Their Effects on Emissions Lecture 36:Hydrocarbon Fuels and Quality Requirements FUELS AND EFFECTS ON ENGINE EMISSIONS
 FUELS AND EFFECTS ON ENGINE EMISSIONS The Lecture Contains: Transport Fuels and Quality Requirements Fuel Hydrocarbons and Other Components Paraffins Cycloparaffins Olefins Aromatics Alcohols and Ethers
FUELS AND EFFECTS ON ENGINE EMISSIONS The Lecture Contains: Transport Fuels and Quality Requirements Fuel Hydrocarbons and Other Components Paraffins Cycloparaffins Olefins Aromatics Alcohols and Ethers
