Annex to the accreditation certificate D-PL according to DIN EN ISO/IEC 17025: and council directives 90/385/EWG 2 and 93/42/EWG 3
|
|
|
- Cecilia James
- 6 years ago
- Views:
Transcription
1 Deutsche Akkreditierungsstelle GmbH Annex to the accreditation certificate D-PL according to DIN EN ISO/IEC 17025: and council directives 90/385/EWG 2 and 93/42/EWG 3 Period of validity: to date of issue: Holder of certificate: GfPS Gesellschaft für Produktionshygiene und Sterilitätssicherung mbh Talbotstrasse 21, Aachen Testing area: Types of tests/test method: Medical devices microbiological-hygienic and physical testing on medical devices including disinfectants, cleaning devices, sterilisation methods as well as sterile barrier- and packaging systems; environmental monitoring Abbreviations used: see last page This document is a translation. The definitive version is the original German annex to the accreditation certificate. 1/8
2 Scope Testing categories Device category Characteristics/ Test options Standard method of testing Microbiological hygienic testing Medical devices Sterility test DIN EN ISO Ph. Eur. 8, USP 38 <71> Total aerobic microbial count Ph. Eur. 8, USP 38 <61> Test for particular microorganisms - Enterobacteriaceae - Salmonellae - Pseudomonas aeruginosa - Staphylococcus aureus - Escherichia coli Test for adequate antimicrobial preservation Ph. Eur. 8, USP 38 <62> SOP 120 Ph. Eur. 8, USP 38 <51> Disinfectants quantitative suspension trials to determine the bactericidal, fungicidal or levurocidal effect (comparison test) of chemical disinfectants and antiseptics (basic testing - Phase 1) detection of the bactericidal, leuvrocidal, fungicidal, tuberculocidal or mycobactericidal efficacy in a quantitative suspension test DIN EN 1040 DIN EN 1275 VAH 9 SOP 134 Period of validity: to Translation - 2/8
3 Testing categories Device category Characteristics/ Test options Standard method of testing Microbiological hygienic testing Sterilisation processes Tests within the context of routine monitoring - by moist heat - by means of biological - by dry heat - by means of biological - by ethylene oxide - by means of biological DIN EN ISO SOP 109 other applicable documents: DIN EN 285 SOP 109 DIN EN ISO SOP 109 Cleaning devices - decontamination systems for mechanically decontaminable mattresses - decontamination systems for bed frames and bedside tables - decontamination systems for circulation containers Tests within the context of routine monitoring - by means of biological - by means of biological - by means of biological SOP 147 Richtlinie des BGA AK-BWA Teil 8 SOP 147 Richtlinie des BGA AK-BWA Teil 8 SOP 147 Richtlinie des BGA AK-BWA Teil 8 Period of validity: to Translation - 3/8
4 Testing categories Device category Characteristics/ Test options Standard method of testing Microbiological hygienic and physical testing Sterile barrier- and packaging systems, packaging materials Test for conformity DIN EN ISO Microbiological barrier DIN Microbiological barrier function using microbiological dusting packaging materials ASTM F 1608 packaging systems SOP 169 (ASTM F 1608) - Accelerated aging ASTM-F Dye-Test ASTM F determination of peel characteristics and seal width - determination of pinholes in plastic laminate ASTM F 3039 DIN EN DIN EN visual inspection ASTM F 1886/F 1886M Period of validity: to Translation - 4/8
5 Environmental Monitoring by Production and Testing of Product Cleanliness according to DIN EN ISO 13485: , Par. 6.4 and Par. 7.5 Microbiological Medical devices Test for bacterial endotoxins Ph. Eur. 8, hygienic testing (LAL-Test) USP 38 <85> Air-conditioning Systems Water and Aqueous Solutions Determination of a population of microorganisms on products DIN EN ISO Clean-room monitoring DIN EN ISO DIN EN ISO DIN EN ISO DIN EN ISO VDI 6022 Paper 1 VDI 2083 Paper 3 Air DIN EN ISO DIN EN ISO DIN VDI 2083 Paper 3 Surfaces DIN EN ISO DIN EN ISO Semi-quantitative microbial enumeration in compressed air Microbiological enumeration test SOP 119 SOP 120 Ph. Eur. 8, USP 38 <61> SOP 120 Regulations 5 DIN EN : DIN EN 1040 : DIN EN 1275 : Packaging for terminally sterilized medical devices. Sealable pouches and reels of porous and plastic film construction. Requirements and test methods Chemical disinfectants and antiseptics - Quantitative suspension test for the evaluation of basic bactericidal activity of chemical disinfectants and antiseptics - Test method and requirements (phase 1) Chemical disinfectants and antiseptics - Quantitative suspension test for the evaluation of basic fungicidal or basic yeasticidal activity of chemical disinfectants and antiseptics - Test method and requirements (phase 1) Period of validity: to Translation - 5/8
6 DIN : Ventilation and air conditioning - Part 4: Ventilation in buildings and rooms of health care DIN EN ISO : Packaging for terminally sterilized medical devices - Part 1: Requirements for materials, sterile barrier systems and packaging systems DIN EN ISO : Sterilization of medical devices - Microbiological methods - Part 1: Determination of a population of microorganisms on products DIN EN ISO : Sterilization of medical devices - Microbiological methods - Part 2: Tests of sterility performed in the definition, validation and maintenance of a sterilization process DIN EN ISO : Cleanrooms and associated controlled environments - Part 1: Classification of air cleanliness by particle concentration DIN EN ISO : Cleanrooms and associated controlled environments - Part 2: Specifications for monitoring and periodic testing to prove continued compliance with ISO DIN EN ISO : Cleanrooms and associated controlled environments - Part 3: Test methods (ISO :2005) DIN EN ISO : Cleanrooms and associated controlled environments - Part 4: Design, construction and start up DIN EN ISO : Cleanrooms and associated controlled environments - Biocontamination control - Part 1: General principles and methods DIN EN ISO : Cleanrooms and associated controlled environments - Biocontamination control - Part 2: Evaluation and interpretation of biocontamination data DIN : AK-BWA ASTM F ASTM F 1886/F1886M-09 ASTM F ASTM F Sterilization - Sterile supply - Part 6: Microbial barrier testing of packaging materials for medical devices which are to be sterilized Working Committee for Bedframe and Trolley Decontamination: mechanical cleaning and disinfection of bedframes, bedside tables, mattresses, trollies and circulatory containers, 1997 Standard Test Method for Microbial Ranking of Porous Packaging Materials (Exposure Chamber Method) Standard Test Method for Determining Integrity of Seals for Flexible Packaging by Visual Inspection Standard Test Method for Detecting Seal Leaks in Porous Medical Packaging by Dye Penetration Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices Period of validity: to Translation - 6/8
7 ASTM F VAH 9 : 2015 Ph. Eur. 8, Ph. Eur. 8, Ph. Eur. 8, Ph. Eur. 8, Ph. Eur. 8, Richtlinie des BGA SOP 109 SOP 119 SOP 120 Standard Test Method for Detecting Leaks in Nonporous Packaging or Flexible Barrier Materials by Dye Penetration detection of the bactericidal, leuvrocidal, fungicidal, tuberculocidal or mycobactericidal efficacy in a quantitative suspension test Test for sterility Microbiological testing of non-sterile products: Total viable aerobic count Microbiological examination of non-sterile products (test for specific micro-organisms) Test for bacterial endotoxins Efficacy of microbial preservation Guidelines from the Federal Ministry for Health for the testing of thermal disinfection processes and cleaning machinery (Complied from the comments on the guidelines of the BGA for the testing of thermal disinfection processes and cleaning machinery, ) Microbiological in process control of EO-/steam-/heat-/ plasmasterilisation Semi-quantitative determination of the microbial count in compressed air Microbiological Differentiation SOP 134 Quantitative suspension test for evaluation of bactericidal and / or fungicidal activity SOP 147 SOP 169 USP 38: 2015, <51> USP 38: 2015, <61> USP 38: 2015, <62> USP 38: 2015, <71> USP 38: 2015, <85> VDI 2083 Paper 3: VDI 6022 Paper 1: Testing of dishwashers, disinfection machine and disinfection facilities for trollies and bedframes using bio Testing of microbial barrier behaviour by microbiological dusting Antimicrobial Effectiveness Test Microbiological Examination of Non-Sterile Products: Microbial Enumeration Tests Microbiological Examination of Non-Sterile Products: Tests for Specified Microorganisms Sterility Tests Bacterial Endotoxins Test Cleanroom technology - Metrology and test methods Hygienic requirements for ventilating and air-conditioning systems and air-handling units, industrial and production sites Period of validity: to Translation - 7/8
8 Abbreviations used (not translated): AK-BWA DIN EN FDA ISO Ph. Eur. SOP USP VDI Arbeitskreis Bettgestell- und Wagendekontamination Deutsches Institut für Normung Europäische Norm Food and Drug Administration International Organization for Standardardization Pharmacopoeia European Standard Operating Procedure (Standardarbeitsanweisung der GfPS) United States Pharmacopeia Verein Deutscher Ingenieure 1 DIN EN ISO/IEC : General requirements for the competence of testing and calibration laboratories 2 Council directive 90/385/EWG des Rates vom 20. Juni 1990 zur Angleichung der Rechtsvorschriften der Mitgliedstaaten über aktive implantierbare medizinische Geräte, ABl. Nr. L 189 vom 20. Juli 1990, S. 17; zuletzt geändert durch Richtlinie 2007/47/EG vom 05. September 2007, ABl. Nr. L 247 vom 21. September 2007, S Council directive 93/42/EWG des Rates vom 14. Juni 1993 über Medizinprodukte, ABl. Nr. L 169 vom 12. Juli 1993, S. 1; zuletzt geändert durch Richtlinie 2007/47/EG vom 05. September 2007, ABl. Nr. L 247 vom 21. September 2007, S DIN EN ISO 13485: Medical devices - Quality management systems - Requirements for regulatory purposes 5 For the transitional periods see the list of harmonized standards on the EU homepage. Period of validity: to Translation - 8/8
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025: and the Directive 93/42/EEC 2 and 90/385/EEC 3
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-13412-01-02 according to DIN EN ISO/IEC 17025:2005 1 and the Directive 93/42/EEC 2 and 90/385/EEC 3 Indefinite since: 10.12.2018
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-13412-01-02 according to DIN EN ISO/IEC 17025:2005 1 and the Directive 93/42/EEC 2 and 90/385/EEC 3 Indefinite since: 10.12.2018
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025: and the Directive 93/42/EEC 2 and 90/385/EEC 3
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-18398-02-02 according to DIN EN ISO/IEC 17025:2005 1 and the Directive 93/42/EEC 2 and 90/385/EEC 3 Period of validity: 26.02.2018
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-18398-02-02 according to DIN EN ISO/IEC 17025:2005 1 and the Directive 93/42/EEC 2 and 90/385/EEC 3 Period of validity: 26.02.2018
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL18398-02-01 according to DIN EN ISO/IEC 17025:2005 Period of validity: 26.02.2018 to 25.02.2023 Holder of certificate: SAL
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL18398-02-01 according to DIN EN ISO/IEC 17025:2005 Period of validity: 26.02.2018 to 25.02.2023 Holder of certificate: SAL
Accreditation. Deutsche Akkreditierungsstelle GmbH hereby confirms that the testing laboratory
 Entrusted pursuant to 8 (1) of the German Accreditation Body Act (AkkStelleG) in conjunction with 1 (1) of the Regulation on the Entrustment of the Accreditation Body (AkkStelleGBV) Accreditation Deutsche
Entrusted pursuant to 8 (1) of the German Accreditation Body Act (AkkStelleG) in conjunction with 1 (1) of the Regulation on the Entrustment of the Accreditation Body (AkkStelleGBV) Accreditation Deutsche
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11272-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 18.10.2017 to 17.10.2022 Holder of certificate: VDZ
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11272-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 18.10.2017 to 17.10.2022 Holder of certificate: VDZ
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-17579-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 07.07.2017 to 06.07.2022 Holder of certificate: gammatest
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-17579-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 07.07.2017 to 06.07.2022 Holder of certificate: gammatest
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-18192-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 15.11.2017 to 14.11.2022 Holder of certificate: ITAP
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-18192-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 15.11.2017 to 14.11.2022 Holder of certificate: ITAP
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-17435-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 06.12.2017 to 08.12.2018 Holder of certificate: k3works
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-17435-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 06.12.2017 to 08.12.2018 Holder of certificate: k3works
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-17435-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 30.09.2016 to 08.12.2018 Holder of certificate: k3works
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-17435-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 30.09.2016 to 08.12.2018 Holder of certificate: k3works
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH German Accreditation Body Annex to the Accreditation Certificate D-PL-20591-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 14.08.2017 to 13.08.2022
Deutsche Akkreditierungsstelle GmbH German Accreditation Body Annex to the Accreditation Certificate D-PL-20591-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 14.08.2017 to 13.08.2022
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11057-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 20.06.2014 to 19.06.2019 Holder of certificate: OELCHECK
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11057-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 20.06.2014 to 19.06.2019 Holder of certificate: OELCHECK
Annex to the Accreditation Certificate D PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D PL 11140 18 00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 06.11.2014 to 05.11.2019 Holder of certificate: Fraunhofer
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D PL 11140 18 00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 06.11.2014 to 05.11.2019 Holder of certificate: Fraunhofer
Hygiene certificate of the mobile shopping trolley cleaning system TROLLEY Wash all in one
 Prof. Dr. R. Mutters Institut für Medizinische Mikrobiologie und Krankenhaushygiene Hans-Meerwein-Str. 2 35043 Marburg Prof. Dr. Mutters Institut Med. Mikrobiologie, Hans-Meerwein-Str. 2, D-35043 Marburg
Prof. Dr. R. Mutters Institut für Medizinische Mikrobiologie und Krankenhaushygiene Hans-Meerwein-Str. 2 35043 Marburg Prof. Dr. Mutters Institut Med. Mikrobiologie, Hans-Meerwein-Str. 2, D-35043 Marburg
Annex to the Accreditation Certificate D PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D PL 18438 02 00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 11.03.2014 to 10.03.2019 Holder of certificate: Jürgen
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D PL 18438 02 00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 11.03.2014 to 10.03.2019 Holder of certificate: Jürgen
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11109-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 13.06.2018 to 22.06.2020 Holder of certificate: TÜV
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11109-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 13.06.2018 to 22.06.2020 Holder of certificate: TÜV
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11065-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 08.02.2016 to 07.02.2021 Holder of certificate: Actemium
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11065-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 08.02.2016 to 07.02.2021 Holder of certificate: Actemium
TÜV RHEINLAND ENERGIE UND UMWELT GMBH. Report on the assessment of the antibacterial properties of AE DeCont rings with AGXX coating
 TÜV RHEINLAND ENERGIE UND UMWELT GMBH Report on the assessment of the antibacterial properties of AE DeCont rings with AGXX coating TÜV report no.: 931/21229709/100 Cologne, 1 October 2015 AE DeCont rings
TÜV RHEINLAND ENERGIE UND UMWELT GMBH Report on the assessment of the antibacterial properties of AE DeCont rings with AGXX coating TÜV report no.: 931/21229709/100 Cologne, 1 October 2015 AE DeCont rings
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11125-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 31.07.2017 to 30.07.2022 Holder of certificate: DIN
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11125-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 31.07.2017 to 30.07.2022 Holder of certificate: DIN
Annex to the Accreditation Certificate D PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D PL 20281 01 00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 31.08.2016 to 30.08.2021 Holder of certificate: Deutsche
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D PL 20281 01 00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 31.08.2016 to 30.08.2021 Holder of certificate: Deutsche
Annex to the Accreditation Certificate D PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D PL 11238 01 00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 09.02.2016 to 08.02.2021 Holder of certificate: ContiTech
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D PL 11238 01 00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 09.02.2016 to 08.02.2021 Holder of certificate: ContiTech
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-19722-01-00 according to DIN EN ISO/IEC 17025:2005 Holder of certificate: Institut für Lacke und Farben Magdeburg ggmbh Fichtestraße
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-19722-01-00 according to DIN EN ISO/IEC 17025:2005 Holder of certificate: Institut für Lacke und Farben Magdeburg ggmbh Fichtestraße
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11035-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 13.02.2018 to 12.02.2023 Holder of certificate: DMT
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11035-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 13.02.2018 to 12.02.2023 Holder of certificate: DMT
Accreditation. a'1" ul. ( oar.hl,.n" Deutsche Akkreditieru ngsstelle G m bh. Y,/.,n" r,nn" Head of Division
 ( oar.hl,.n" Akkreditieru ngsstel le Deutsche Akkreditieru ngsstelle G m bh Entrusted according to Section 8 subsection 1 AkkStelleG in connection with Section 1 subsection 1 AkkStellecBV Signatory the
( oar.hl,.n" Akkreditieru ngsstel le Deutsche Akkreditieru ngsstelle G m bh Entrusted according to Section 8 subsection 1 AkkStelleG in connection with Section 1 subsection 1 AkkStellecBV Signatory the
10.6 Schedule of Accreditation
 Testing Laboratory is accredited by the GCC Accreditation Center () in accordance with the recognised International Standard, General requirements for the competence of testing and calibration laboratories
Testing Laboratory is accredited by the GCC Accreditation Center () in accordance with the recognised International Standard, General requirements for the competence of testing and calibration laboratories
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-18137-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 19.03.2018 to 18.03.2023 Holder of certificate: Continental
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-18137-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 19.03.2018 to 18.03.2023 Holder of certificate: Continental
Chemical disinfectants and antiseptics - Hygienic handwash - Test method and requirements (phase 2/step 2)
 Irish Standard Chemical disinfectants and antiseptics - Hygienic handwash - Test method and requirements (phase 2/step 2) CEN 2013 No copying without NSAI permission except as permitted by copyright law.
Irish Standard Chemical disinfectants and antiseptics - Hygienic handwash - Test method and requirements (phase 2/step 2) CEN 2013 No copying without NSAI permission except as permitted by copyright law.
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-18869-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 30.08.2013 to 29.08.2018 Holder of certificate: ilf
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-18869-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 30.08.2013 to 29.08.2018 Holder of certificate: ilf
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-17453-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 03.07.2017 to 02.07.2022 Holder of certificate: SPIE
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-17453-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 03.07.2017 to 02.07.2022 Holder of certificate: SPIE
6/18/2015. USP <800> Update. USP <800> - What is it? What are HDs? John Voliva, R.Ph. Director of Legislative Relations PCCA
 USP Update John Voliva, R.Ph. Director of Legislative Relations PCCA USP - What is it? Proposed Chapter Published April 2014, Revised and Re-Published December 2014 Covers the handling of hazardous
USP Update John Voliva, R.Ph. Director of Legislative Relations PCCA USP - What is it? Proposed Chapter Published April 2014, Revised and Re-Published December 2014 Covers the handling of hazardous
Process Filtration From Pure to Sterile
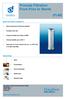 From Pure to Sterile MAIN FEATURES & BENEFITS: High mechanical and thermal stability Excellent flow rate Integrity testable according to HIMA Thermal stability up to 200 C Approved for Food Contact Use
From Pure to Sterile MAIN FEATURES & BENEFITS: High mechanical and thermal stability Excellent flow rate Integrity testable according to HIMA Thermal stability up to 200 C Approved for Food Contact Use
<800> Hazardous Drugs Handling in Healthcare Settings. Jeanne Sun, PharmD
 Hazardous Drugs Handling in Healthcare Settings Jeanne Sun, PharmD Purpose of Approximately 8 million healthcare workers in the United States are potentially exposed to hazardous drugs (HDs)
Hazardous Drugs Handling in Healthcare Settings Jeanne Sun, PharmD Purpose of Approximately 8 million healthcare workers in the United States are potentially exposed to hazardous drugs (HDs)
Percent Change in Seal Strength Relative to the Control for Maximum Load ASTM F88
 Percent Change in Seal Strength Relative to the Control for Maximum Load ASTM F88 Upper -15-10 -5 0 5 10 15 Percent Change = Mean (Test-Control)/Mean(Control)*100 Percent Change in Seal Strength Relative
Percent Change in Seal Strength Relative to the Control for Maximum Load ASTM F88 Upper -15-10 -5 0 5 10 15 Percent Change = Mean (Test-Control)/Mean(Control)*100 Percent Change in Seal Strength Relative
Process Filtration From Pure to Sterile
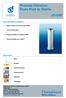 From Pure to Sterile MAIN FEATURES & BENEFITS: Highly robust and mechanically stable Very durable design Integrity testable according to HIMA Thermal stability up to 200 C INDUSTRIES: Dairy Brewery Food
From Pure to Sterile MAIN FEATURES & BENEFITS: Highly robust and mechanically stable Very durable design Integrity testable according to HIMA Thermal stability up to 200 C INDUSTRIES: Dairy Brewery Food
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 eutsche Akkreditierungsstelle Gmb Annex to the Accreditation Certificate -PL-19399-01-00 according to IN EN ISO/IEC 17025:2005 ate of issue: 29.11.2018 older of certificate: Inspectorate eutschland Gmb
eutsche Akkreditierungsstelle Gmb Annex to the Accreditation Certificate -PL-19399-01-00 according to IN EN ISO/IEC 17025:2005 ate of issue: 29.11.2018 older of certificate: Inspectorate eutschland Gmb
This document is a preview generated by EVS
 EESTI STANDARD EVS-EN 1040:2006 Keemilised desinfektsioonivahendid ja antiseptikumid. Bakteritsiidne põhitoime. Katsemeetodid ja nõuded (faas 1) Chemical disinfectants and antiseptics - Quantitative suspension
EESTI STANDARD EVS-EN 1040:2006 Keemilised desinfektsioonivahendid ja antiseptikumid. Bakteritsiidne põhitoime. Katsemeetodid ja nõuded (faas 1) Chemical disinfectants and antiseptics - Quantitative suspension
Process Filtration From Pure to Sterile
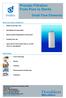 From Pure to Sterile MAIN FEATURES & BENEFITS: Ready-to-use filter units Sterilizable and regenerable Highly durable Polypropylene construction Excellent flow rate Approved for Food Contact Use acc. to
From Pure to Sterile MAIN FEATURES & BENEFITS: Ready-to-use filter units Sterilizable and regenerable Highly durable Polypropylene construction Excellent flow rate Approved for Food Contact Use acc. to
Cytotoxic Safety Cabinets ENVAIR eco safe Plus
 Cytotoxic Safety Cabinets ENVAIR eco safe Plus Class Cytotoxic Standard Width (m) II Safety EN 12469 0.9 / 1.2 Cabinet DIN 12980 1.5 / 1.8 Clean technology for a clean environment ENVAIR eco safe Plus
Cytotoxic Safety Cabinets ENVAIR eco safe Plus Class Cytotoxic Standard Width (m) II Safety EN 12469 0.9 / 1.2 Cabinet DIN 12980 1.5 / 1.8 Clean technology for a clean environment ENVAIR eco safe Plus
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11321-02-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 20.12.2016 to 19.12.2021 Holder of certificate: TÜV
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11321-02-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 20.12.2016 to 19.12.2021 Holder of certificate: TÜV
This document is a preview generated by EVS
 EUROPEAN PRESTANDARD PRÉNORME EUROPÉENNE EUROPÄISCHE VORNORM ENV 807 May 2001 ICS 71.100.50 Supersedes ENV 807:1993 English version Wood preservatives - Determination of the effectiveness against soft
EUROPEAN PRESTANDARD PRÉNORME EUROPÉENNE EUROPÄISCHE VORNORM ENV 807 May 2001 ICS 71.100.50 Supersedes ENV 807:1993 English version Wood preservatives - Determination of the effectiveness against soft
User Guide. Pall Laboratory Manifold. For laboratory use. Not for use in a manner other than indicated. Introduction. Regulatory References
 www.pall.com/lab User Guide Pall Laboratory Manifold For laboratory use. Not for use in a manner other than indicated. Introduction Application and Intended Use The membrane filtration (MF) technique is
www.pall.com/lab User Guide Pall Laboratory Manifold For laboratory use. Not for use in a manner other than indicated. Introduction Application and Intended Use The membrane filtration (MF) technique is
Process Filtration From Pure to Sterile
 From Pure to Sterile MAIN FEATURES & BENEFITS: Highly robust and mechanically stable Very durable design Integrity testable according to HIMA Thermal stability up to 200 C Approved for Food Contact Use
From Pure to Sterile MAIN FEATURES & BENEFITS: Highly robust and mechanically stable Very durable design Integrity testable according to HIMA Thermal stability up to 200 C Approved for Food Contact Use
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-20258-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 2016-02-02 to 2021-02-01 Holder of certificate: Amphenol-Tuchel
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-20258-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 2016-02-02 to 2021-02-01 Holder of certificate: Amphenol-Tuchel
P-SRF V STERILE AIR PLEATED DEPTH FILTER ELEMENTS
 P-SRF V STERILE AIR PLEATED DEPTH FILTER ELEMENTS Compressed Air & Process Filtration The P-SRF V filter element is a sterile grade, pleated depth filter element in a stainless-steel body. The improved
P-SRF V STERILE AIR PLEATED DEPTH FILTER ELEMENTS Compressed Air & Process Filtration The P-SRF V filter element is a sterile grade, pleated depth filter element in a stainless-steel body. The improved
SKN_N0106AnnexJ_R2. Annex J - Specific requirements for PVT collector Certification. 1. Introduction and definition
 Annex J - Specific requirements for PVT collector Certification 1. Introduction and definition PVT collectors (named hybrid collectors in ISO 9806) generate heat and electric power at the same time. They
Annex J - Specific requirements for PVT collector Certification 1. Introduction and definition PVT collectors (named hybrid collectors in ISO 9806) generate heat and electric power at the same time. They
SafeFAST Top. Class II Microbiological Safety Cabinets PROTECTION, SAFETY, RELIABILITY. AND MORE.
 Class II Microbiological Safety Cabinets PROTECTION, SAFETY, RELIABILITY. AND MORE. Class II Microbiological Safety Cabinets SAFETY CABINETS WITH AUTOMATIC REGULATION AND MICROPROCESSOR BASED MONITORING
Class II Microbiological Safety Cabinets PROTECTION, SAFETY, RELIABILITY. AND MORE. Class II Microbiological Safety Cabinets SAFETY CABINETS WITH AUTOMATIC REGULATION AND MICROPROCESSOR BASED MONITORING
Sterilisering - Ångautoklaver - Stora autoklaver Sterilization - Steam sterilizers - Large sterilizers
 Sterilisering - Ångautoklaver - Stora autoklaver Sterilization - Steam sterilizers - Large sterilizers Sterilization - Steam sterilizers Large sterilizers Sterilisering - Ångautoldaver - Stora autoldaver
Sterilisering - Ångautoklaver - Stora autoklaver Sterilization - Steam sterilizers - Large sterilizers Sterilization - Steam sterilizers Large sterilizers Sterilisering - Ångautoldaver - Stora autoldaver
Sound-absorbing spacer Sorp10
 Sponsor: Reference number: 7.3 / 27080 This document is only a translated version; not legally attested Max Frank GmbH & Co. KG Mitterweg 1 94339 Leiblfing Federal Institute for Materials Research and
Sponsor: Reference number: 7.3 / 27080 This document is only a translated version; not legally attested Max Frank GmbH & Co. KG Mitterweg 1 94339 Leiblfing Federal Institute for Materials Research and
CHEMICAL DISINFECTANTS - QUANTITATIVE SUSPENSION TEST FOR THE EVALUATION OF VIRUCIDAL ACTIVITY AGAINST BACTERIOPHAGES OF
 IRISH STANDARD I.S. EN 13610:2003 ICS 11.080.20 67.050 71.100.35 CHEMICAL DISINFECTANTS - QUANTITATIVE SUSPENSION TEST FOR National Standards Authority of Ireland Dublin 9 Ireland Tel: (01) 807 3800 Tel:
IRISH STANDARD I.S. EN 13610:2003 ICS 11.080.20 67.050 71.100.35 CHEMICAL DISINFECTANTS - QUANTITATIVE SUSPENSION TEST FOR National Standards Authority of Ireland Dublin 9 Ireland Tel: (01) 807 3800 Tel:
RSB List of Documents and references
 Type of document: Reference Document Date: 20 March 2017 RSB List of Documents and references RSB reference code: [RSB-DOC-10-001] Published by the Roundtable on Sustainable Biomaterials. This publication
Type of document: Reference Document Date: 20 March 2017 RSB List of Documents and references RSB reference code: [RSB-DOC-10-001] Published by the Roundtable on Sustainable Biomaterials. This publication
SafeFAST Top. Class II Microbiological Safety Cabinets PROTECTION, SAFETY, RELIABILITY. AND MORE.
 SafeFAST Top Class II Microbiological Safety Cabinets PROTECTION, SAFETY, RELIABILITY. AND MORE. OUR COMMITMENTS New technologies for a low environmental impact Fully aware that our choices of today will
SafeFAST Top Class II Microbiological Safety Cabinets PROTECTION, SAFETY, RELIABILITY. AND MORE. OUR COMMITMENTS New technologies for a low environmental impact Fully aware that our choices of today will
Latest publications September 2013
 Latest publications September 2013 'Latest publications' lists the European Standards (EN), CEN Workshop Agreements (CWA), CEN Technical Specifications (CEN/TS) and CEN Technical Reports (CEN/TR) that
Latest publications September 2013 'Latest publications' lists the European Standards (EN), CEN Workshop Agreements (CWA), CEN Technical Specifications (CEN/TS) and CEN Technical Reports (CEN/TR) that
Annex to the Accreditation Certificate D-K according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-K-15102-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 05.07.2017 to 04.07.2022 Date of issue: 05.07.2017
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-K-15102-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 05.07.2017 to 04.07.2022 Date of issue: 05.07.2017
PROBE LOCKER DIGEST MEETS BS 4680:1996. We reserve the right to alter specifications without prior consent.
 P R BE locker digest We reserve the right to alter specifications without prior consent. INDEX Probe products have been manufactured, to the highest standards, in the UK since 1960 We have been engineering
P R BE locker digest We reserve the right to alter specifications without prior consent. INDEX Probe products have been manufactured, to the highest standards, in the UK since 1960 We have been engineering
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 15380 Third edition 2016-12-01 Lubricants, industrial oils and related products (class L) Family H (Hydraulic systems) Specifications for hydraulic fluids in categories HETG,
INTERNATIONAL STANDARD ISO 15380 Third edition 2016-12-01 Lubricants, industrial oils and related products (class L) Family H (Hydraulic systems) Specifications for hydraulic fluids in categories HETG,
DÜRR NDT GmbH & CO. KG Höpfigheimer Straße Bietigheim-Bissingen
 Evaluation of the image quality of a radiographic film emulsion AGFA D7 exposed by X-rays and developed by machine processing (8 min cycle time at 29 C) using Dürr NDT XR D-6 NDT developer and XR F-6 NDT
Evaluation of the image quality of a radiographic film emulsion AGFA D7 exposed by X-rays and developed by machine processing (8 min cycle time at 29 C) using Dürr NDT XR D-6 NDT developer and XR F-6 NDT
Period of validity: to Date of issue:
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11190-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 09.03.2017 to 02.02.2020 Holder of certificate: TÜV
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11190-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 09.03.2017 to 02.02.2020 Holder of certificate: TÜV
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-20575-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 2018-03-05 to 2021-07-28 Holder of certificate: innogy
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-20575-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 2018-03-05 to 2021-07-28 Holder of certificate: innogy
ČSN EN ISO ČSN ISO 4832
 Testing laboratory working sites: 1 Praha 2 Brno Areál Slatina, Tuřanka 115, 627 00 Brno The laboratory is qualified to provide expert opinions and to interpret test results. The Laboratory is qualified
Testing laboratory working sites: 1 Praha 2 Brno Areál Slatina, Tuřanka 115, 627 00 Brno The laboratory is qualified to provide expert opinions and to interpret test results. The Laboratory is qualified
TECHINCAL DESCRIPTION BIO BAN
 TECHINCAL DESCRIPTION BIO BAN LAF CABINET ARTICLE DESCRIPTION CODE BIOBAN 48 00303400000 BIOBAN 72 00303500000 ST-[BIOBAN]_EN-0 Rev. 0 of 16/06/2010 Introduction Designed for the situation where is requested
TECHINCAL DESCRIPTION BIO BAN LAF CABINET ARTICLE DESCRIPTION CODE BIOBAN 48 00303400000 BIOBAN 72 00303500000 ST-[BIOBAN]_EN-0 Rev. 0 of 16/06/2010 Introduction Designed for the situation where is requested
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate DPL110551000 according to DIN EN ISO/IEC 17025:2005 Period of validity: 20140612 to 20160711 Date of issue: 20140612 Holder of
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate DPL110551000 according to DIN EN ISO/IEC 17025:2005 Period of validity: 20140612 to 20160711 Date of issue: 20140612 Holder of
Skyddshandskar mot kemikalier och mikroorganismer
 SVENSK STANDARD SS-EN 374-1:2004 Fastställd 2004-01-23 Utgåva 2 Skyddshandskar mot kemikalier och mikroorganismer Del 1: Terminologi och fordringar på prestanda Protective gloves against chemicals and
SVENSK STANDARD SS-EN 374-1:2004 Fastställd 2004-01-23 Utgåva 2 Skyddshandskar mot kemikalier och mikroorganismer Del 1: Terminologi och fordringar på prestanda Protective gloves against chemicals and
RSB ROUNDTABLE ON SUSTAINABLE BIOMATERIALS RSB List of Documents and references. 18 January RSB reference code: RSB-DOC
 RSB ROUNDTABLE ON SUSTAINABLE BIOMATERIALS RSB List of Documents and references 18 January 2019 RSB reference code: RSB-DOC-10-001 Published by the Roundtable on Sustainable Biomaterials. This publication
RSB ROUNDTABLE ON SUSTAINABLE BIOMATERIALS RSB List of Documents and references 18 January 2019 RSB reference code: RSB-DOC-10-001 Published by the Roundtable on Sustainable Biomaterials. This publication
Introducing Zep MicroSolve TM. Now Microorganisms Have No Place to Hide
 Introducing Zep MicroSolve TM Now Microorganisms Have No Place to Hide Eliminate the Possibility of Cross-Contamination. Removes biofilm and kills its microorganisms, such as Listeria, E. coli, and Salmonella,
Introducing Zep MicroSolve TM Now Microorganisms Have No Place to Hide Eliminate the Possibility of Cross-Contamination. Removes biofilm and kills its microorganisms, such as Listeria, E. coli, and Salmonella,
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 DEKRA IST RELIABILITY SEVICES INC. No. 22 Puding Rd., East Dist. Hsinchu City 300, Taiwan, R.O.C. Phone: 886 3 579 5766 ext 7100 Mr. General Lee general.lee@dekra-ist.com
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 DEKRA IST RELIABILITY SEVICES INC. No. 22 Puding Rd., East Dist. Hsinchu City 300, Taiwan, R.O.C. Phone: 886 3 579 5766 ext 7100 Mr. General Lee general.lee@dekra-ist.com
USP 800: Quick Summary
 SCSHP 2014 Fall Meeting Why USP 800: Quick Summary Natasha Nicol, Pharm D, FASHP Director, Medication Safety Cardinal Health Chapter builds on the standards established by existing compounding chapters
SCSHP 2014 Fall Meeting Why USP 800: Quick Summary Natasha Nicol, Pharm D, FASHP Director, Medication Safety Cardinal Health Chapter builds on the standards established by existing compounding chapters
EBI 12 The new data logger generation
 EBI 12 The new data logger generation 3 EBI 12 The new data logger generation High quality stainless steel housing Application range from -90 C to 150 C High temperature accuracy up to 0.05 C Extended
EBI 12 The new data logger generation 3 EBI 12 The new data logger generation High quality stainless steel housing Application range from -90 C to 150 C High temperature accuracy up to 0.05 C Extended
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 TUV SUD CANADA INC. 1229 Ringwell Drive Newmarket, Ontario, Canada, L3Y 8T8 William (Mac) Elliott Phone: 813 284 2736 melliott@tuvam.com www.tuv-sud.ca ELECTRICAL
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 TUV SUD CANADA INC. 1229 Ringwell Drive Newmarket, Ontario, Canada, L3Y 8T8 William (Mac) Elliott Phone: 813 284 2736 melliott@tuvam.com www.tuv-sud.ca ELECTRICAL
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CONSUMER TESTING LABORATORIES, INC. 611 Dream Valley Road Rogers, AR 72756 Chessie Koko Phone: 479-636-8782 CHEMICAL Valid To: May 31, 2018 Certificate Number:
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CONSUMER TESTING LABORATORIES, INC. 611 Dream Valley Road Rogers, AR 72756 Chessie Koko Phone: 479-636-8782 CHEMICAL Valid To: May 31, 2018 Certificate Number:
Schedule of Accreditation issued by United Kingdom Accreditation Service 2 Pine Trees, Chertsey Lane, Staines-upon-Thames, TW18 3HR, UK
 (part of Smithers Rapra and Ltd) Packaging Technical Services Cleeve Road Leatherhead Surrey KT22 7RU Contact: Mr G Collis Tel: +44 (0)1372 802000 Fax: +44 (0)1372 802245 E-Mail: gcollis@smithers.com Website:
(part of Smithers Rapra and Ltd) Packaging Technical Services Cleeve Road Leatherhead Surrey KT22 7RU Contact: Mr G Collis Tel: +44 (0)1372 802000 Fax: +44 (0)1372 802245 E-Mail: gcollis@smithers.com Website:
STORAGE DESIGN LIMITED TEL: PROBE LOCKER DIGEST
 P R B E locker digest We reserve the right to alter specifications without prior consent. STORAGE DESIGN LIMITED Tel: 01446 772614 INDEX Probe products have been manufactured, to the highest standards,
P R B E locker digest We reserve the right to alter specifications without prior consent. STORAGE DESIGN LIMITED Tel: 01446 772614 INDEX Probe products have been manufactured, to the highest standards,
National IMPLEMENTING MEASURES (IM) related to the specific General Protocol requirement*
 Authority: BaFin (DE) National IMPLEMENTING MEASURES (IM) related to the specific General Protocol requirement* Article 23 of the Methodology for Peer Review: National implementing measures may include
Authority: BaFin (DE) National IMPLEMENTING MEASURES (IM) related to the specific General Protocol requirement* Article 23 of the Methodology for Peer Review: National implementing measures may include
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 11140-4 First edition 2001-05-01 Sterilization of health care products Chemical indicators Part 4: Class 2 indicators for steam penetration test packs Stérilisation des produits
INTERNATIONAL STANDARD ISO 11140-4 First edition 2001-05-01 Sterilization of health care products Chemical indicators Part 4: Class 2 indicators for steam penetration test packs Stérilisation des produits
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11068-09-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 10.07.2014 to 09.07.2019 Holder of certificate: Karlsruher
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-11068-09-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 10.07.2014 to 09.07.2019 Holder of certificate: Karlsruher
Test Report. CHTC JIAHUA NONWOVEN CO.,LTD No.99,WaGou New Village, Pengchang,Xiantao,Hubei, CHINA
 Test Report Order No.: 832/2 Page of 6 Client : CHTC JIAHUA NONWOVEN CO.,LTD No.99,WaGou New Village, Pengchang,Xiantao,Hubei, CHINA Date of order: 5 May 28 Receipt of sample material: May 28 Origin of
Test Report Order No.: 832/2 Page of 6 Client : CHTC JIAHUA NONWOVEN CO.,LTD No.99,WaGou New Village, Pengchang,Xiantao,Hubei, CHINA Date of order: 5 May 28 Receipt of sample material: May 28 Origin of
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 DURHAM HOLDINGS CORPORATION INC. DBA MME TESTING 1039 Industrial Court Suwanee, GA 30024 Danny Collazo Phone: 678 730 2000 MECHANICAL Valid To: October 31,
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 DURHAM HOLDINGS CORPORATION INC. DBA MME TESTING 1039 Industrial Court Suwanee, GA 30024 Danny Collazo Phone: 678 730 2000 MECHANICAL Valid To: October 31,
DOWNLOAD OR READ : EN ISO 105 B06 XNCQAY PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : EN ISO 105 B06 XNCQAY PDF EBOOK EPUB MOBI Page 1 Page 2 en iso 105 b06 xncqay en iso 105 b06 pdf en iso 105 b06 xncqay en. Format Language; std 1 88: PDF std 2 88: Paper CHF 88; Buy
DOWNLOAD OR READ : EN ISO 105 B06 XNCQAY PDF EBOOK EPUB MOBI Page 1 Page 2 en iso 105 b06 xncqay en iso 105 b06 pdf en iso 105 b06 xncqay en. Format Language; std 1 88: PDF std 2 88: Paper CHF 88; Buy
Deutsche Akkreditierungsstelle GmbH
 (( DAk~~"h' Akkreditierungsstelle Deutsche Akkreditierungsstelle GmbH Entrusted according to Section 8 subsection 1 AkkStelleG in connection with Section 1 subsection 1 AkkStelleGBV Signatory to the Multilateral
(( DAk~~"h' Akkreditierungsstelle Deutsche Akkreditierungsstelle GmbH Entrusted according to Section 8 subsection 1 AkkStelleG in connection with Section 1 subsection 1 AkkStelleGBV Signatory to the Multilateral
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CONSUMER TESTING LABORATORIES, INC. 2601 SE Otis Corley Drive Bentonville, AR 72712 Stephanie Anderson Phone: 479 286 2376 MECHANICAL Valid To: September 30,
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 CONSUMER TESTING LABORATORIES, INC. 2601 SE Otis Corley Drive Bentonville, AR 72712 Stephanie Anderson Phone: 479 286 2376 MECHANICAL Valid To: September 30,
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 EXPONENT, INC.¹ 3440 Market Street Suite 600 Philadelphia, PA 19104 Ryan Siskey Phone: 215 594 8896 Email: rsiskey@exponent.com MECHANICAL Valid To: June 30,
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 EXPONENT, INC.¹ 3440 Market Street Suite 600 Philadelphia, PA 19104 Ryan Siskey Phone: 215 594 8896 Email: rsiskey@exponent.com MECHANICAL Valid To: June 30,
DEPYROGENATING TUNNELS
 DEPYROGENATING TUNNELS DEPYROGENATING TUNNELS STERILINE ST series laminar flow depyrogenating tunnels have been designed to depyrogenize ampoules, vials and syringes with a continuos process maintaining
DEPYROGENATING TUNNELS DEPYROGENATING TUNNELS STERILINE ST series laminar flow depyrogenating tunnels have been designed to depyrogenize ampoules, vials and syringes with a continuos process maintaining
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 NATIONAL EXPOSURE TESTING, INC. 3211 Centennial Road Sylvania, OH 43560 Christa Lammers Phone: 419 841 1065 MECHANICAL Valid To: October 31, 2018 Certificate
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 NATIONAL EXPOSURE TESTING, INC. 3211 Centennial Road Sylvania, OH 43560 Christa Lammers Phone: 419 841 1065 MECHANICAL Valid To: October 31, 2018 Certificate
Chemical indicators and test devices Benchmarking sterilisation processes.
 Chemical indicators and test devices Benchmarking sterilisation processes www.stericlin.com Regulatory landscape National Law Directive / Regulation Guidelines Standards 2 Directive Regulation Law Guideline
Chemical indicators and test devices Benchmarking sterilisation processes www.stericlin.com Regulatory landscape National Law Directive / Regulation Guidelines Standards 2 Directive Regulation Law Guideline
Annex to the Accreditation Certificate D IS according to DIN EN ISO/IEC 17020:2012
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D IS 14153 02 01 according to DIN EN ISO/IEC 17020:2012 Period of validity: 16.04.2015 to 14.04.2020 Date of issue: 16.04.2015
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D IS 14153 02 01 according to DIN EN ISO/IEC 17020:2012 Period of validity: 16.04.2015 to 14.04.2020 Date of issue: 16.04.2015
DEUTSCHE NORM DIN 8077
 DEUTSCHE NORM DIN 877 September 28 D ICS 23.4.2 Supersedes DIN 877:27-5 Polypropylene (PP) pipes PP-H, PP-B, PP-R, PP-RCT Dimensions Rohrus Polypropylen (PP) PP-H, PP-B, PP-R, PP-RCT Maße Normen-Download-Beuth-Türk
DEUTSCHE NORM DIN 877 September 28 D ICS 23.4.2 Supersedes DIN 877:27-5 Polypropylene (PP) pipes PP-H, PP-B, PP-R, PP-RCT Dimensions Rohrus Polypropylen (PP) PP-H, PP-B, PP-R, PP-RCT Maße Normen-Download-Beuth-Türk
DEUTSCHE NORM July 2004 DIN EN ISO 9038 { Test for sustained combustibility of liquids (ISO 9038 : 2002) English version of DIN EN ISO 9038
 DEUTSCHE NORM July 2004 DIN EN ISO 9038 { ICS 13.220.40; 87.040 Test for sustained combustibility of liquids (ISO 9038 : 2002) English version of DIN EN ISO 9038 Prüfung der Weiterbrennbarkeit von Flüssigkeiten
DEUTSCHE NORM July 2004 DIN EN ISO 9038 { ICS 13.220.40; 87.040 Test for sustained combustibility of liquids (ISO 9038 : 2002) English version of DIN EN ISO 9038 Prüfung der Weiterbrennbarkeit von Flüssigkeiten
Accredited competence for measurements in railway operations: Aerodynamics Test Laboratory. DB Systemtechnik
 Accredited competence for measurements in railway operations: Aerodynamics Test Laboratory DB Systemtechnik Our products Test expertise: for rolling stock, components, infrastructure and interfaces As
Accredited competence for measurements in railway operations: Aerodynamics Test Laboratory DB Systemtechnik Our products Test expertise: for rolling stock, components, infrastructure and interfaces As
DEUTSCHE NORM June Plastics Symbols and abbreviated terms Part 1: Basic polymers and their special characteristics (ISO : 2001)
 DEUTSCHE NORM June 2002 Plastics Symbols and abbreviated terms Part 1: Basic polymers and their special characteristics (ISO 1043-1 : 2001) English version of DIN EN ISO 1043-1 { EN ISO 1043-1 ICS 01.040.83;
DEUTSCHE NORM June 2002 Plastics Symbols and abbreviated terms Part 1: Basic polymers and their special characteristics (ISO 1043-1 : 2001) English version of DIN EN ISO 1043-1 { EN ISO 1043-1 ICS 01.040.83;
The Continuing Evolution of Food Grade Lubricants
 The Continuing Evolution of Food Grade Lubricants By Jim Girard, Vice President and Chief Marketing Officer LUBRIPLATE Lubricants Co. / Fiske Brothers Refining Co. Newark, New Jersey and Toledo, Ohio For
The Continuing Evolution of Food Grade Lubricants By Jim Girard, Vice President and Chief Marketing Officer LUBRIPLATE Lubricants Co. / Fiske Brothers Refining Co. Newark, New Jersey and Toledo, Ohio For
Envair Lab SCS Evo Comfort+ Class II Microbiological Safety Cabinets
 Envair Lab SCS Evo Comfort+ Class II Microbiological Safety Cabinets Envair SCS Evo Comfort + Class II Microbiological Safety Cabinets set the benchmark in laminar airflow technology, creating a new generation
Envair Lab SCS Evo Comfort+ Class II Microbiological Safety Cabinets Envair SCS Evo Comfort + Class II Microbiological Safety Cabinets set the benchmark in laminar airflow technology, creating a new generation
EU TOY DIRECTIVE 2009/48/EC: OVERVIEW - REGULATORY CONTEXT AND MAJOR CHANGES
 EU TOY DIRECTIVE 2009/48/EC: OVERVIEW - REGULATORY CONTEXT AND MAJOR The EU Toy Directive was revised in order to take into account the new technological developments and increasing child safety requirements.
EU TOY DIRECTIVE 2009/48/EC: OVERVIEW - REGULATORY CONTEXT AND MAJOR The EU Toy Directive was revised in order to take into account the new technological developments and increasing child safety requirements.
This document is a preview generated by EVS
 EESTI STANDARD EVS-EN 14832:2005 Petroleum and related products - Determination of the oxidation stability and corrosivity of fire-resistant phosphate ester fluids Petroleum and related products - Determination
EESTI STANDARD EVS-EN 14832:2005 Petroleum and related products - Determination of the oxidation stability and corrosivity of fire-resistant phosphate ester fluids Petroleum and related products - Determination
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that Charter Coating Service (2000) Ltd. Suite
CERTIFICATE OF ACCREDITATION ANSI-ASQ National Accreditation Board 500 Montgomery Street, Suite 625, Alexandria, VA 22314, 877-344-3044 This is to certify that Charter Coating Service (2000) Ltd. Suite
Clarigard Filters High-performance filters for the clarification and prefiltration of process fluids
 Lenntech info@lenntech.com www.lenntech.com Tel. +31-1-261.9. Fax. +31-1-261.62.89 Data Sheet Clarigard Filters High-performance filters for the clarification and prefiltration of process fluids Clarigard
Lenntech info@lenntech.com www.lenntech.com Tel. +31-1-261.9. Fax. +31-1-261.62.89 Data Sheet Clarigard Filters High-performance filters for the clarification and prefiltration of process fluids Clarigard
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 NATIONAL EXPOSURE TESTING, INC. 3211 Centennial Road Sylvania, OH 43560 Christa Lammers Phone: 419 841 1065 MECHANICAL Valid To: October 31, 2016 Certificate
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 NATIONAL EXPOSURE TESTING, INC. 3211 Centennial Road Sylvania, OH 43560 Christa Lammers Phone: 419 841 1065 MECHANICAL Valid To: October 31, 2016 Certificate
USP 800: Now What Do We Have To Do?!
 SCSHP 2014 Fall Meeting USP 800: Now What Do We Have To Do?! Natasha Nicol, Pharm D, FASHP Director, Medication Safety Cardinal Health Objectives Explain the reasoning behind USP 800 and how it expands
SCSHP 2014 Fall Meeting USP 800: Now What Do We Have To Do?! Natasha Nicol, Pharm D, FASHP Director, Medication Safety Cardinal Health Objectives Explain the reasoning behind USP 800 and how it expands
Josephus Paulus Clemens Maria van Doornmalen Gomez Hoyos. Dutch Norm Committee and Dutch delegate in EN TC 102 WG 2 Testing EN TC WG 3 Requirements
 Steam penetration Josephus Paulus Clemens Maria van Doornmalen Gomez Hoyos March 2012 Dutch Norm Committee and Dutch delegate in EN TC 102 WG 2 Testing EN TC WG 3 Requirements EN TC WG 5 Small steam sterilizers
Steam penetration Josephus Paulus Clemens Maria van Doornmalen Gomez Hoyos March 2012 Dutch Norm Committee and Dutch delegate in EN TC 102 WG 2 Testing EN TC WG 3 Requirements EN TC WG 5 Small steam sterilizers
ASTM Consensus Standards The Latest in Packaging Testing. Presented by: Dhuanne Dodrill President Rollprint Packaging Products, Inc.
 ASTM Consensus Standards The Latest in Packaging Testing Presented by: Dhuanne Dodrill President Rollprint Packaging Products, Inc. ASTM Overview Established in 1898 Voluntary, Full-Consensus Standards
ASTM Consensus Standards The Latest in Packaging Testing Presented by: Dhuanne Dodrill President Rollprint Packaging Products, Inc. ASTM Overview Established in 1898 Voluntary, Full-Consensus Standards
Annex to the Accreditation Certificate D-PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-18436-01-01 according to DIN EN ISO/IEC 17025:2005 Period of validity: 22.01.2018 to 28.10.2019 Holder of certificate: ISP
Deutsche Akkreditierungsstelle GmbH Annex to the Accreditation Certificate D-PL-18436-01-01 according to DIN EN ISO/IEC 17025:2005 Period of validity: 22.01.2018 to 28.10.2019 Holder of certificate: ISP
The Path to Package Integrity
 The Path to Package Integrity Outline Package integrity logic and thought process. Establishing quality standards. Evaluation of common methodologies. Alternative methodologies. Driving forward with package
The Path to Package Integrity Outline Package integrity logic and thought process. Establishing quality standards. Evaluation of common methodologies. Alternative methodologies. Driving forward with package
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z
 SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 METTLER TOLEDO (INDUSTRIAL) 1900 Polaris Parkway Columbus, OH 43240 Frank Gardone Phone: 412 213 2263 CALIBRATION Valid To: April 30,
SCOPE OF ACCREDITATION TO ISO/IEC 17025:2005 & ANSI/NCSL Z540-1-1994 METTLER TOLEDO (INDUSTRIAL) 1900 Polaris Parkway Columbus, OH 43240 Frank Gardone Phone: 412 213 2263 CALIBRATION Valid To: April 30,
